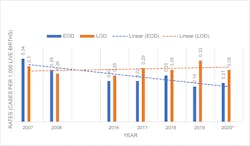
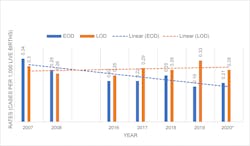
Decline in GBS early onset disease rates is likely to continue
The updated 2010 CDC guidelines and 2020 American Society of Microbiology (ASM) guidelines for detection and identification of Group B Streptococcus (GBS) in conjunction with the changes made in 2019 by the American College of Obstetricians and Gynecologists (ACOG) and American Academy of Pediatrics (AAP) and other groups have favorably contributed to the decline of cases of GBS early-onset disease (EOD). Since 2008, EOD has been on a downward trend, while late-onset disease (LOD) has remained at a higher flatter trajectory. 1-5 (Figure 1)
The most current CDC Active Bacterial Surveillance (ABC’s) Report, Emerging Infections Program Network for Group B Streptococcus (2019), estimates the national incidence of EOD at 0.19 cases per 1,000 live births and the incidence of LOD at 0.33 cases per 1,000 live births. EOD is defined as cases occurring at less than 7 days old, while LOD are cases between 7 and 89 days old.7 EOD is preventable with IAP, which means that almost half of all infants with GBS disease cases in the United States are preventable by the current available strategy.
In 2019, ACOG updated its guidance to include an updated recommendation to now perform routine screening for group B Streptococcus from the vagino-rectal swab between 36-0/7 and 37-6/7 weeks of gestation. Previously, the CDC’s perinatal GBS guidelines recommended the prenatal GBS screening be performed between 35-0/7 and 36-6/7 weeks gestation. This change has two main advantages. First, it covers the majority of full-term deliveries until week 41. Secondly, given that GBS colonization can be transient, testing later in the pregnancy (within five weeks before delivery) reduces the chance of the colonization status having changed before delivery.2 It has been shown that increased intervals between screening and delivery decrease the positive predictive value (PPV) for GBS cultures, especially when the interval exceeds six weeks.3
Reduction in the incidence of EOD has been directly associated with the implementation and compliance with these revised guidelines, yet there is still room for improvement. The guidelines recognize that prenatal screening using a nucleic acid amplification test (NAAT) provides an increase in sensitivity when compared to culture. Studies have shown that 81%-83% of infants born with EOD were born to mothers with a negative screening test result by culture.2,8 Using NAAT increases the opportunity to identify additional mothers that are at risk of passing GBS infection to the baby, resulting in treatment of the GBS colonized mother with appropriate antibiotic during labor. Unfortunately, it is known that not all laboratories have implemented the use of NAAT for GBS screening. A study published in 2018 shows that out of 507 laboratories only 18.7% used GBS NAAT, with 7.3% using GBS NAAT for antenatal screening only, 4.1% for intrapartum screening only, and 3.4% for both antenatal and intrapartum testing.9
What are the benefits of NAAT testing compared to culture for prenatal screening?
Molecular tests in general have proven to be more sensitive than traditional culture, and they also have other added benefits. One advantage of NAAT is the ability to detect non-hemolytic strains of GBS. It is estimated that 5%–6% of the GBS isolates are non-hemolytic strains and can be missed when using traditional culture, and/or if using certain chromogenic media.2 For the most part, hemolytic strains are associated with virulence, and non-hemolytic strains of GBS are often thought to be virulence attenuated. A recent study showed that non-hemolytic, non-pigmented strains of GBS isolated from septic neonates can exhibit hypervirulence.10 The ASM guidelines emphasize the importance of detecting both hemolytic and non-hemolytic strains when using isolation methods for GBS.
NAAT platforms allow for workflow efficiencies and decreased hands-on processing time. Molecular tests provide definitive results in less time than culture, without depending on viable organisms to detect the presence of the bacteria. For many tests, the pre-analytical phase is as important as the test itself, but for culture, a proper sample collection and transport is a must to obtain optimal results.
Following the broth enrichment step, which is recommended by the guidelines for all samples, NAAT can provide a much quicker turnaround time to results compared to culture. The average turnaround time of some automated molecular platforms ranges between 60–90 min. In comparison, culture will take an additional 24–48 hrs. of incubation time and may require additional biochemical tests to confirm any suspect colonies.
Many clinical microbiology laboratories are understaffed, and many are losing expertise with personnel leaving and/or retiring from the workforce. Laboratories must find ways to be more efficient and do more with less. Under these circumstances, a molecular platform can be a viable solution. Many molecular platforms are moderately complex, which allows the test to be performed in any area of the laboratory. The results don’t require any interpretation, and in many instances, the results can be printed or downloaded, minimizing transcription errors. Depending on the molecular platform being used, NAAT can provide the laboratory flexibility and ease of integration to their workflow.
What are the shortfalls of NAAT for GBS screening?
The one challenge that laboratories encounter when using molecular tests is the absence of the organism if and/or when susceptibility testing is needed for patients allergic to penicillin or cefazolin. Additionally, due to increased rates of clindamycin resistance, confirmation of isolate susceptibility is needed before the use of these antibiotics. In these instances, the laboratory needs to have the ability to go back to the enrichment broth and set up a subculture to agar media. There are laboratories that set up culture plates at the same time as they run the molecular test in the event that additional antibiotic sensitivity testing is required. Laboratories rely on the ordering physician to properly identify patients with penicillin allergies on the requisition forms. It is recommended that GBS screening orders include an indication of penicillin allergy as a mandatory field in electronic orders.
Moving forward, decline in EOD is likely to continue.
In theory, there should be a continued decline in the incidence rate of GBS EOD supported, in part, by the current ACOG/AAP guidelines, which are intended to improve the PPV for GBS colonization during delivery given that the recommended window for screening has been moved to be closer to delivery and covers a higher percentage of the births. Additionally, increased adoption of NAAT over culture by the laboratories for prenatal screening will result in enhanced accuracy of the diagnosis of GBS-positive mothers who, in turn, will be identified to receive intrapartum antibiotic prophylaxis to avoid transmission of GBS to their newborn during labor.
References:
- Verani JR, McGee L, Schrag SJ. Prevention of Perinatal Group B Streptococcal Disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010; 59(RR-10):1-36.
- Filkins L, Hauser J, Robinson-Dunn B, et al. Guidelines for the Detection and Identification of Group B Stretpococcus, American Society of Microbiology (Initially posted March 10, 2020, updated July 23, 2021). https://asm.org/Guideline/Guidelines-for-the-Detection-and-Identification-of Accessed 5/2/2022.
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns, ACOG Committee Opinion Number 797, Obstetrics & Gynecology 2020 Feb; 135(2);e51-72. doi: 10.1097/AOG.0000000000003669.
- Puopolo KM, Lynfield R, Cummings JJ. Management of Infant at Risk for Group B Streptococcal Disease, Pediatrics 2019 Aug; 144 (2); 1-17. doi: 10.1542/peds.2019-1881.
- ABCs Bact Facts Interactive Data Dashboard | CDC , reviewed June 29, 2021. https://www.cdc.gov/abcs/bact-facts-interactive-dashboard.html Accessed 5/2/2022.
- Group B Strep: Fast Facts and Statistics | CDC , reviewed June 11, 2020. https://www.cdc.gov/groupbstrep/about/fast-facts.html Accessed 5/2/2022.
- CDC Active Bacterial Core (ABC) Surveillance Report, Group B Streptococcus 2019, reviewed July 19, 2021. https://www.cdc.gov/abcs/reports-findings/surv-reports.html reviewed Accessed 5/2/2022.
- Nanduri SA, Petit S, Smelser C, et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015, JAMA Pediatrics 2019 Mar; 173(3): 224-233. doi: 10.1001/jamapediatrics.2018.4826.
- Fay K, Almendras O, Robinson-Dunn B, Schrag S. Antenatal and Intrapartum Nucleic Acid Amplification Test Use for Group B Streptococcus Screening – United States 2016, J Diag Micro & Inf Dis 2019 Jun, vol 94:2; 157-159. doi: 10.1016/j.diagmicrobio.2018.11.026.
- Gendrin C, Vornhagen J, Armistead B, et al. A Nonhemolytic Group B Streptococcus Strain Exhibits Hypervirulence, Journal of Infectious Diseases Brief Report 2018 Mar; 217:983-987. doi: 10.1093/infdis/jix646.
Frances C. Esteve has more than 40 years of experience in IVD at Meridian Bioscience and is currently a Marketing Product Manager. She has held positions in product research and development, manufacturing, international sales, and as a ASQC Certified Quality Auditor. She received her Bachelor of Biological Sciences from the University of Cincinnati.
About the Author

Frances C. Esteve
has more than 40 years of experience in IVD at Meridian Bioscience and is currently a Marketing Product Manager. She has held positions in product research and development, manufacturing, international sales, and as a ASQC Certified Quality Auditor. She received her Bachelor of Biological Sciences from the University of Cincinnati.