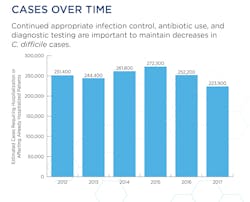
Mitigating the risks of C. diff infections and antimicrobial resistance
Antibiotic treatment options for suspected and confirmed C. difficile infections (CDI) have clinicians taking more time to consider the increased risks of antimicrobial resistance (AMR) potentially faced by the patient in the future. As a fairly common hospital-acquired infection (HAI) with an estimated 200,000 cases diagnosed each year according to the Mayo Clinic, CDIs have become the subject of debate when it comes to treating the infections with antibiotics. While treatment decisions made today may address a current CDI, clinicians are also forced to consider a patient’s potential for future AMR as well.
An “opportunistic” pathogen
With its basis in gut microbiota disruption, C. difficile appears to lie in wait for a healthy gut to become disrupted, putting the patient at risk of a CDI that requires additional treatment.
According to David M. Lyerly, Chief Science Officer at TechLab, “Clostridioides difficile is a prime example of an opportunistic intestinal pathogen. This anaerobic pathogen can germinate and grow in the large intestine when our gut microbiota has been depleted. Without competition from our healthy microbiota, C. difficile spores germinate and when growing as vegetative cells, produce toxins that can cause life-threatening colitis due to mucosal damage and severe uncontrolled inflammation. The spores can persist long after the symptoms of C. difficile infection (CDI) have resolved, probably months to years in some patients, explaining why some patients relapse repeatedly after CDI.”
He added, “Any disruption of the gut microbiota – whether by antibiotics, chemotherapy, proton pump inhibitors, other gastrointestinal infections or conditions – can result in CDI and can lead to C. difficile colonization in asymptomatic persons. Probably the most striking and thoroughly studied are the high number of hospitalized patients who carry C. difficile but who remain asymptomatic. These patients meet the description of a “carrier” as defined in the 2016 ESCMID guideline for the diagnosis of C. difficile disease.”
Explaining the most common route of acquiring a CDI, Ted E. Schutzbank, PhD, D(ABMM), Science Affairs Manager at Meridian Bioscience, pointed out, “Acquisition of C. difficile in the hospital setting due to poor handwashing practices and poor disinfection of hospital rooms between patients is the most common route, as the C. difficile spores can persist for months in the hospital environment. In addition, asymptomatic colonization of individuals with C. difficile has been demonstrated. Various studies have shown asymptomatic colonization of between 0-17.5 percent depending on the population group studied; colonization with toxigenic strains ranges from 1-5 percent.”
Continuing, Schutzbank noted that “prevalence in healthy individuals is low, but rises significantly in various populations, especially in adults that have had contact with healthcare settings, and the elderly in long-term healthcare facilities or nursing homes. Healthcare workers have also been shown to have a higher prevalence for asymptomatic C. difficile colonization. Neonates in the first four weeks of life have also been shown to be asymptomatically colonized, but such colonization clears as a healthy gut microbiome is established.”
Antibiotic use risk factors
At the heart of debate over antibiotic use with CDIs remains the issue of the potential for an increased risk of AMR with every C. difficile infection that a patient is treated for. Many clinicians assert that the more antibiotics that are prescribed as treatment for CDIs, the higher the risk they will have a lower treatment effect in subsequent or future CDIs. This issue has clinicians and laboratorians alike looking to find an acceptable way to treat CDIs while mitigating the level of AMR risk involved for patients.
Looking at treatment options for symptomatic and asymptomatic patients, Rodney C. Arcenas, PhD, D(ABMM), Director of Clinical Sciences, Microbiology, at Roche Molecular Systems, said “There is little debate about treating symptomatic persons, with the IDSA 2018 guidelines recommending the use of vancomycin or fidaxomicin over metronidazole for initial episodes of CDI. Metronidazole may be used in settings where vancomycin or fidaxomicin may be limited. The treatment choices are similar for fulminant CDI and recurrent CDI, with the potential for fecal microbiota transplantation recommended only for multiple recurrences of CDI in patients who have failed appropriate antibiotic treatments. There have been instances of increased MICs of metronidazole from C. difficile clinical isolates.”
He added, “The utility of antibiotics in asymptomatic and/or known carriers of C. difficile remains a topic of debate. Although it is recognized that carriers may present a level of risk to themselves for the development of CDI and also as a potential reservoir of transmission, the benefit of eradicating any C. difficile carriage is, so far, unproven. There are other confounding factors to consider in asymptomatic C. difficile carriers, such as the use of potential offending antibiotics, age, use of proton pump inhibitors, healthcare environmental exposure, underlying disease such as ulcerative colitis, and immune status, which have all been shown to contribute to the development of CDI.”
Lyerly summed up, “CDI most often is triggered by an antibiotic and ironically, the therapy for this antibiotic-associated disease is another antibiotic. CDI can be triggered by most antibiotics, although some seem to be more closely associated with the disease, perhaps because of increased use of those particular antibiotics. CDI most often is treated with metronidazole, vancomycin, or fidaxomicin, and fortunately, most patients respond well to treatment.
In recent years, metronidazole has not been prescribed as much as vancomycin for CDI patients. Fidaxomicin is the newest antibiotic for CDI and has lower relapse rates than those associated with vancomycin. Although any of these three antibiotics can effectively treat CDI, they also can kill much of the normal gut microbiota. This is a concern because if a patient is incorrectly diagnosed with CDI and receives inappropriate treatment with any of these antibiotics, the patient can be predisposed to true CDI.”
Schutzbank also pointed out some of the more common antibiotics used to treat CDIs and their potential for resistance, saying. “C. difficile is known to be resistant to a broad range of antibiotics. The most common antibiotics associated with CDI are ampicillin, amoxicillin, cephalosporins, clindamycin, and fluoroquinolones. The most common choice of antibiotics for treatment of CDI include metronidazole, vancomycin, and fidaxomycin. The use of metronidazole has essentially been discontinued due to the high rate of resistance to this antibiotic. According to the current IDSA C. difficile guidelines metronidazole is no longer recommended as first-line therapy for adults.”
He added, “Resistance of C. difficile to vancomycin has also been reported, although the levels of resistance vary appreciably in different areas of the world and depend on which resistance break-point system is used. A sentinel survey in the U.S. found that 17.9 percent of C. difficile isolates were resistant to vancomycin based on the EUCAST breakpoints. This suggests a serious problem for the use of vancomycin should the rates of resistance increase in the future. Indeed, in Israel, one strain of C. difficile, ribotype 27, with reduced susceptibilities to both metronidazole and vancomycin now predominates. For this reason, appropriate testing, following the published guidelines by the IDSA as well as other organizations, must be rigorously followed to ensure a proper diagnosis of CDI, since overdiagnosis due to false positive results will result in inappropriate antibiotic therapy for those patients, which has long-term consequences for the development of antibiotic resistance.”
Looking for other options
As the debate continues about the use – and potential overuse – of antibiotics for C. difficile infections, clinicians remind the lab industry of the challenges they face in finding an acceptable option that will not only treat the CDI, but also help maintain antimicrobial susceptibility for the patient in the future.
Arcenas reported, “Interestingly, the topic of screening asymptomatic patients for C. difficile has been considered as a means of identifying carriers upon admission into the hospital.1 In a recent study, patients being admitted for surgical services were screened for the carriage of C. difficile by collecting perirectal swabs and positive patients were flagged in their system. Treatment for C. difficile was considered only if they became symptomatic for CDI. The study authors stated they observed a decrease in CDI rates during this 10-month period of screening compared to before this intervention period where no screening was performed. It should be noted that the IDSA guidelines state that there is insufficient data to recommend screening for asymptomatic carriage and implementing contact precautions at this time. However, the data is promising as a means of controlling CDI in healthcare institutions.”
He continued, “Additionally, healthcare institutions can institute mechanisms to control the rates of CDI in their institution with an antibiotic stewardship program that has input from the clinical laboratory, pharmacy, infection control, and infectious disease clinicians. Currently, the IDSA strongly recommends minimizing or restricting the use of high-risk antibiotics (fluoroquinolones, clindamycin, and cephalosporins) and the total number of antibiotics prescribed.”
Also asserting the benefits of antimicrobial stewardship, Lyerly summarizes, “Diagnostic testing for CDI continues to be challenging, and laboratories have to weigh the benefits and limitations of the various types of tests now available. Toxin testing provides the highest positive predictive values, but concerns have been raised about the lower sensitivity. GDH and NAAT assays provide higher sensitivity but detect the organism and not toxin. NAAT assays offer the highest sensitivity for the organism but over-diagnose patients who are colonized and who carry spores.”
He added, “For these reasons, guidelines have recommended algorithm testing that brings together the advantages of these tests when multiple tests are implemented. The strength of this approach is built on the ability to determine if a patient is carrying C. difficile and whether a patient’s diarrhea is caused by C. difficile. By using this approach, CDI patients are more accurately diagnosed, inappropriate treatment is minimized, and importantly, antimicrobial stewardship is practiced.”
References:
- Linsenmeyer K, O’Brien W, Brecher S, Strymish J, Rochman A, Itani K, Gupta K. Clostridium difficile screening for colonization during an outbreak setting, Clin Infect Dis. 67(12): 1912–1914, https://doi.org/10.1093/cid/ciy455