New tools combat a complex antimicrobial resistance problem
Earning CEUs
For a printable version of the August CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1) Describe the causes of antimicrobial resistance.
2) Recall the five objectives of the global action plan on antimicrobial resistance.
3) Describe the role of diagnostic assays in providing information to help providers use antibiotics judiciously and effectively and cite an example of an assay.
4) Describe the three new programs launched this year to combat antimicrobial resistance.
Antimicrobial resistance (AMR) is among the greatest threats to the health and well-being of the world’s population. If present trends continue, AMR will become a greater cause of mortality than heart disease or cancer by 2050.1 As the bacteria that cause infections become increasingly drug resistant, even common medical procedures canbecome more life-threatening for patients.
This is not a theoretical future risk; it is already happening. In 2019, the U.S. Centers for Disease Control and Prevention (CDC) released updated estimates for the toll of drug-resistant infections in the United States, demonstrating that the risk is greater than previously believed, affecting more than 2.8 million patients annually and leading to more than 35,000 deaths.2
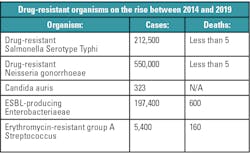
Antimicrobials are a mainstay of modern medicine but decades of outmoded prescribing practices and extensive use of antimicrobials in food production have driven a rise in organisms that are resistant to these life-saving drugs. The CDC has identified 18 antibiotic-resistant organisms, including Drug-resistant Salmonella Serotype Typhi, Drug-resistant Neisseria gonorrhoeae, Candida auris, ESBL-producing Enterobacteriaceae, and Erythromycin-resistant group A Streptococcus.2
The increased prevalence of drug-resistant organisms is impacting patients in both community and hospital settings. The World Health Organization (WHO) found widespread prevalence globally of patients with antibiotic-resistant infections, as well as wide variation in the rate of occurrence, depending on the country. For example, the WHO said 8 percent to 65 percent of urinary tract infections are not treatable with a regularly prescribed antibiotic. And among patients with bloodstream infections, the percentage with resistant infections ranged from zero to 82 percent.3
It is critical to understand the patient impact of drug-resistant infections to drive improved prescribing practices and behaviors. One example is the case of Tatiana Vargas,4 a member of the Antimicrobial Resistance Fighter Coalition, which aims to drive behavioral change by elevating patient stories. In the case of Vargas, her story emphasizes that drug-resistant infections can happen when least expected. She was a healthy, recently married newlywed living in California. After returning home from her honeymoon, she began to feel unwell. She went to an emergency room and was misdiagnosed with a strep infection, treated with antibiotics, and sent home.
In no time, the infection moved to her lungs and Tatiana landed in the ICU. Different doctors then diagnosed her condition as methicillin-resistant S. aureus (MRSA), a very difficult staph infection for which the initial antibiotic no longer worked. After quarantine and many weeks of treatment, she was released. Tatiana now lives with a chronic cough and the reality that the infection might return.
While much of the focus on AMR has highlighted the need for a renewed pipeline of new antimicrobials, experts including the World Health Organization (WHO) and CDC recognize the need for a multi-pronged approach. In May 2015, the World Health Assembly (WHA) adopted a global action plan on antimicrobial resistance, which outlines five objectives:
- Improving awareness and understanding of antimicrobial resistance through effective communication, education, and training.
- Strengthening the knowledge and evidence base through surveillance and research.
- Reducing the incidence of infection through effective sanitation, hygiene and infection prevention measures.
- Optimizing the use of antimicrobial medicines in human and animal health.
- Developing the economic case for sustainable investment that considers the needs of all countries and increases investment in new medicines, diagnostic tools, vaccines and other interventions.
The main goal of the global action plan is to ensure, for as long as possible, continuity of successful treatment and prevention of infectious diseases with effective and safe medicines that are quality-assured, used in a responsible way, and accessible to all who need them.5
The same multi-pronged approach has been applied to combat SARS-CoV-2, the virus that causes COVID-19. The COVID-19 pandemic has reinforced the importance of effective measures to control the virus, which also help curb antimicrobial resistance, and these include:
- Improving education on the importance of hygiene in communities and infection prevention and control in healthcare facilities to contain spread of COVID-19.
- Accessing and utilizing surveillance data to identify and monitor infection outbreaks, spread and trends, both at a population level and within healthcare facilities and systems.
- Scaling-up COVID-19 diagnostic testing to identify and isolate people who are infected. Patients who are infected with COVID-19 and critically ill are vulnerable to secondary bacterial infections and drug resistance, and therefore, the importance of identifying a cause of infection – viral, bacterial, fungal, parasitic – is critical in order to optimize treatment.
While the urgent and immediate threat of the novel COVID-19 virus is the utmost priority for governments, health ministries, public health agencies and hospital systems, we also need to ensure that actions to curb and prevent AMR continue. The COVID-19 pandemic has been an acute shock to the world as the novel coronavirus has affected the health of millions and the lives and livelihood of billions. But the renewed focus on public health and the investments made to respond to the pandemic can have lasting impact to the long-term effort to address the untreatable infections caused by AMR.
By resetting efforts towards the five objectives outlined in the AMR Global Action Plan, we can ensure continuity of successful treatment and prevention of infectious diseases with effective and safe medicines. The first objective is to improve awareness and understanding of antimicrobial resistance through effective communication, education and training. By deploying training and awareness programs, the remaining four objectives will be able to be achieved more effectively.
Over the past year, three new programs were launched focused on training and awareness building. These programs provide relevant information and training for healthcare and non-healthcare audiences, with effective training tools that enhance knowledge on ways to combat AMR within the United States and around the world.
Training for infection prevention
The Society for Healthcare Epidemiology of America (SHEA) has developed and launched a new training course on best practices in infection prevention and control for hospital clinicians with direct patient care responsibilities. The online program, Prevention Course in HAI Knowledge and Control (Prevention CHKC), is supported by an educational grant from BD (Becton, Dickinson and Company) and was announced at SHEA’s Spring Meeting in Boston (2019).8
“With many healthcare facilities stretched to or beyond their limits and others preparing to be, this course provides critical information and skills to keep frontline providers, their families and patients safe in this crisis,” said Hilary Babcock, MD, MPH, chair of the SHEA Education and Research Foundation. “These prevention processes are not necessarily intuitive, and the need for refreshing these skills among healthcare workers is high in normal times and critical during this global pandemic.”
While efforts to reduce healthcare-associated infections (HAIs) have been effective for certain infection types within specific care settings, HAIs still affect one in every 20 hospitalized patients, leading to morbidity, mortality, and excess healthcare costs.9 One cause may be persistent gaps between recommended infection prevention and control strategies and what is practiced.
SHEA developed the Prevention CHKC program to address these knowledge gaps and improve the safety and quality of patient care provided by frontline healthcare personnel. The program includes 12 online, interactive modules authored by a program committee of multidisciplinary experts. Currently, they are focused on healthcare in acute care settings, such as hospitals, with the aim for the content to be adaptable for other care settings.
The training will help frontline personnel improve their understanding of their roles in HAI prevention. The program focuses on:
- Performing basic prevention strategies of hand hygiene and cleaning of the environment and equipment.
- Recognizing and managing outbreaks.
- Understanding strategies to prevent common HAIs, including central line-associated bloodstream infections, catheter-associated urinary tract infections, surgical site infections, ventilator-associated events, C. difficile infections, and methicillin-resistant S. aureus (MRSA).
- Developing performance and accountability measures to assess infection prevention practices.
Prevention CHKC is intended for frontline healthcare personnel and is defined for the purposes of this course as individuals in healthcare facilities who are “responsible for direct patient care.” This can include doctors, nurses, pharmacists, laboratory technicians, and others involved in supporting infection prevention and antimicrobial stewardship programs. The course offers practical resources to improve infection prevention, and can be used during planned training, e.g. new employee training or scheduled training events, as well as when a healthcare team needs a refresher on a specific topic. Prevention CHKC provides up to 6.75 AMA PRA Category 1 Credit, ABIM MOC Points, ACPE Pharmacy Credits, and Nursing Contact Hours. As of early June 2020, close to 600 participants have successfully completed the course.
While the course typically costs $25 for non-SHEA members, the organization is offering the course free to everyone through December 31, 2020.
Training to scale up diagnostics
Guiding appropriate use of antimicrobials relies on the accurate diagnosis of infection. Unfortunately, diagnostic tests are frequently unavailable or underutilized, resulting in antimicrobials being prescribed empirically and often inappropriately. Increasing awareness of the role of diagnostic testing can help laboratories to report timely, critical results to clinicians and antibiotic stewardship teams. The data generated from diagnostic testing also informs local, national, and global infectious disease surveillance systems. When combined with robust antimicrobial stewardship programs, rapid diagnostics have provided shorter times to optimal therapy, shorter hospital lengths of stay, and lower hospital costs.10,11
Director General of the World Health Organization Tedros Adhanom Ghebreyesus, PhD, has been quoted as saying, “An accurate diagnosis is the first step to getting effective treatment. No one should suffer or die because of a lack of diagnostic services, or because the right tests were not available.”12
The London School of Hygiene and Tropical Medicine partnered with BD and global experts to develop a Massive Open Online Course (MOOC) to raise awareness, knowledge and to advocate for the role of diagnostics in the AMR response.13 This partnership was formed in direct response to a call for public-private private partnerships in the global fight against AMR at the 2016 World Economic Forum in Davos, Switzerland. The MOOC aims to increase awareness of the role of diagnostics to guide the appropriate use of antibiotics in clinical medicine, screen for resistant infections in healthcare settings, and for surveillance to monitor AMR trends and the effectiveness of stewardship interventions.
An example of the depth that participants gain learning about each module is seen when Healthcare-Associated Infections (HAIs) are introduced in week three. HAIs are infections that patients acquire in healthcare settings, including hospitals and long-term care facilities. Organisms that cause HAIs such as MRSA (Methicillin-resistant S. aureus), CPO (Carbapenemase-producing organisms), VRE (Vancomycin-resistant Enterococci), and C. diff (Clostridoides difficile) can colonize in a patient or cause an infection in a patient. Based on the source of the clinical sample and the type of test completed, the diagnostic result will provide clear information about the possible colonization or infection. The role of diagnostic testing in identifying and facilitating the prevention and control of these HAIs is important.
The MOOC goes into detail on these tests and how the results impact individual patient management, healthcare system level surveillance, and global surveillance. For CPO, the MOOC describes the role of diagnostics for resistant organisms. They can be used to screen for carriers to enable infection prevention and control measures, to identify infected individuals to guide treatment or for active surveillance of resistant HAIs to enable alerts of outbreaks. CPOs can be detected using molecular testing, which has the benefit of fast time-to-results and can be more sensitive but more expensive. CPOs can also be detected using culture on plated media, which can take up to three days and may lack sensitivity, but it is less expensive than molecular testing. Once the samples are received in the laboratory, they can be processed and have a molecular assay completed. If tests – such as BD Phoenix CPO Detect, Carba NP, or modified Hodge test – are used, the patient sample needs to plated and incubated, with the testing completed on colony growth.
At the completion of the six-week course, participants are able to describe the top causes of AMR (linked to WHO and CDC priority list of pathogens) – such as Gram-negative bacterial pathogens causing urinary tract infections (UTI) and healthcare associated infections (HAIs), sepsis and gonorrhea – and explain the role of diagnostics for these conditions. They understand that everyone has a role to play in the fight against AMR – both for now and the future.
This course is intended for health professionals, such as clinicians, nurses, nurse-practitioners, pharmacists, lab managers, technicians, faculty, and students. The course is offered free of charge and is a flexible means of providing quality educational content from a community of experts to a global audience.
First offered in September 2019, the MOOC has reached more than 10,000 learners worldwide. “It is our vision that a world of knowledge and resources related to diagnostics and AMR be made available to healthcare workers all over the world with a simple click of a key on a computer. We believe that this knowledge will advance the ability to detect the emergence of resistance and take quick action.” says Renuka Gadde, Vice President, BD Global Health.
Increased awareness of drug-resistant infections
To change behaviors and practices around antibiotic utilization, the risk of drug-resistant infections needs to be better understood by organizations and individuals. Antimicrobial resistance is a complex problem that involves human health, animal management and agriculture practices. A report published by Wellcome Trust14 found that public understanding of antimicrobial resistance and its impact is currently limited. Some of reasons for the lack of understanding cited were the use of technical language and jargon, a lack of urgency in messaging, and a lack of understanding that AMR is a universal problem that affects everyone. Increasing awareness is the first objective of Global Action Plan and is a critical element in combatting AMR.
As mentioned earlier, the Antimicrobial Resistance Fighter Coalition was formed to address the need for greater awareness about the problem.15 It is a collective of like-minded organizations, leaders, and individuals united in their commitment to address the threat and burden of antimicrobial resistance. With members from 44 countries, they share their work and commitments to inspire others. The global campaign works to substantially increase awareness of AMR and encourage action across a wide range of stakeholders, including policymakers, health agency officials, professional societies, clinicians, patients and family members.
The coalition focuses its educational efforts on the key elements needed to combat AMR, which include:
- Infection prevention, like vaccines and handwashing, to reduce the number of infections and the need to utilize antibiotics.
- Improved utilization of current diagnostic tests and new tests to provide rapid diagnosis of the causative agents.
- New treatments to provide targeted, effective management of infections.
As mentioned above, the Coalition also amplifies the voices of patients from around the world and reminds us that AMR can impact anyone at any time. Take, for example, the case of Lisa Smith,16 a new mother from Arkansas who acquired a multidrug-resistant wound infection during childbirth. Lisa’s drug-resistant infection required multiple readmissions, six weeks of antibiotic treatment and home healthcare treatment from an advanced wound center for 15 weeks. The emotional costs of spending her maternity leave fighting a drug-resistant infection instead of bonding with her newborn child were substantial.
The Antimicrobial Resistance Fighter Coalition features a website and social media channels that provide educational tools, current articles and publications, and current events focused on general AMR information, infection prevention diagnostic stewardship, and antibiotic stewardship. The website directly links to information provided by the coalition’s partner organizations. It features an interactive map that allows viewers to identify and see messages of Antimicrobial Resistance Fighters from around the world. It is designed to be relevant and appealing both to people who are learning about AMR for the first time, as well as those who are already very knowledgeable about this threat.
In conclusion
Continued education to increase awareness of drug resistance infections will drive change in how we use our current antibiotics. Training on infection prevention and diagnostic stewardship will help mitigate the overuse of our current antibiotics. We are all living in a truly unique time when citizens throughout the world are now keenly awareness of the risks of untreatable infections and the effect they can have on the global community. The COVID-19 pandemic has changed how we all think about public health and will result in appreciation among policymakers for the importance of investing in robust public health and healthcare delivery systems. Among these to include substantially strengthened infectious disease prevention capabilities, and within the general public, the need to engage in safer hygienic practices.
References:
- The AMR Review. Tackling drug-resistant infections globally: final report and recommendations. 2016.
- Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019.
- World Health Organization. (2018). High levels of antibiotic resistance found worldwide, new data shows. Retrieved from https://www.who.int/mediacentre/news/releases/2018/antibiotic-resistance-found/en/ Accessed June 22, 2020.
- Tatiana Vargas, Antimicrobial Resistance Fighter Coalition message. Antimicrobial Resistance Fighter Coalition. https://antimicrobialresistancefighters.org/stories/story-tatiana-chiprez-vargas Accessed June 22, 2020.
- Global Action Plan. World Health Organization (WHO). 2015. https://www.who.int/antimicrobial-resistance/global-action-plan/en/ Accessed June 19, 2020.
- Weiner LM, Fridkin SK, Aponte-Torres Z, et al. Vital Signs: Preventing Antibiotic-Resistant Infections in Hospitals — United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65:235–241.
- Arefian H, Vogel M, Kwetkat A, Hartmann M. Economic Evaluation of Interventions for Prevention of Hospital Acquired Infections: A Systematic Review Zeeb H, ed. PLoS ONE. 2016 https://doi.org/10.1371/journal.pone.0146381
- Prevention Chkc. Learning CE SHEA Online Education Center. https://learningce.shea-online.org/content/prevention-chkc#group-tabs-node-course-default1 Accessed June 22, 2020.
- Haque H., et al. 2018. Healthcare-associated infections – an overview. Infect Drug Resist Rev 11: 2321-2333.
- Beganovic M et al. 2017. Effect of matrix-assisted laser desorption ionization - Time of flight mass spectrometry (MALDI-TOF MS) alone versus MALDI-TOF MS combined with real-time antimicrobial stewardship interventions on time to optimal antimicrobial therapy in patients with positive blood cultures. Journal of Clinical Microbiology (2017) 55:5 (1437-1445).
- Morency-Potvin P, Schwartz DN, Weinstein RA. 2017. Antimicrobial stewardship: how the microbiology laboratory can right the ship. Clin Microbiol Rev 30:381– 407.
- First-ever WHO list of essential diagnostic tests to improve diagnosis and treatment outcomes. May 2018. WHO, Press Release. https://www.who.int/news-room/detail/15-05-2018-first-ever-who-list-of-essential-diagnostic-tests-to-improve-diagnosis-and-treatment-outcomes. Accessed June 10, 2020.
- The role of diagnostics in the antimicrobial resistance response. London School of Hygiene & Tropical Medicine. https://www.lshtm.ac.uk/study/courses/short-courses/free-online-courses/diagnostics-in-amr-response Accessed June 22, 2020.
- Framing Resistance: The Framing Toolkit. Wellcome Trust. 2020. http://www.wellcome.ac.uk/reframing-resistance
- Antimicrobial Resistance Fighter Coalition. https://antimicrobialresistancefighters.org/ Accessed June 22, 2020.
- Lisa Smith, Antimicrobial Resistance Fighter Coalition message. Antimicrobial Resistance Fighter Coalition. https://antimicrobialresistancefighters.org/stories/story-lisa-smith?category=532
About the Author

Diane Flayhart, MBA
is the Global Program Leader for Antimicrobial Resistance at BD. Flayhart works to champion and support development of new Antimicrobial Resistance (AMR) program initiatives in global awareness, infection prevention/control and antimicrobial stewardship. Flayhart leads efforts for the Antimicrobial Resistance Fighter Coalition, which is mobilized by BD.