Procalcitonin testing as an aid to antibiotic stewardship
Earning CEUs:
For a printable version of the September CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
SEPTEMBER LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss campaigns that are aimed to provide guidance on effect antibiotic stewardship programs.
2. Describe the limitations in testing and diagnosis for bacterial sepsis.
3. Recall the utility of PCT testing in diagnosis and therapy in patients with sepsis.
4. Discuss conclusions of various studies of PCT testing in antibiotic stewardship programs and the importance of strict adherence to published algorithms for the best therapy outcomes.
Early detection and treatment of serious bacterial infections, sepsis, and septic shock—with appropriate antibiotic and supportive therapy—is critical to patient survival. Delaying antibiotic therapy increases the risk of increasing illness severity and the overall risk of mortality. Because the stakes are so high, the Surviving Sepsis Campaign (SSC) guidelines, organized by the Society of Critical Care Medicine in 2002, strongly recommend intravenous administration of empiric, broad spectrum antibiotics as soon as possible after recognition and collection of blood and site-specific cultures, with a goal of administration within one hour of either sepsis of septic shock.1
The challenge, however, lies in the fine act of balancing antibiotic delivery with antibiotic stewardship. Bacterial sepsis symptoms are nonspecific and can be mistaken for many other serious conditions, such as trauma, diabetic ketoacidosis, acute pancreatitis, and myocardial infarction.2 Additionally, approximately 40 percent of patients have only vague symptoms, such as weakness or pain, and geriatric patients—who have the highest rates of sepsis—frequently have atypical symptoms that can be misconstrued as stroke, dementia, or even simple dehydration.2,3 Finally, culture results typically may not be available for several days and have a low rate of success: Site cultures are negative in 20 to 47 percent of patients with severe sepsis and only five to 10 percent of blood cultures are positive.4-6 Consequently, when sepsis or a serious infection such as pneumonia that carries a high risk of progressing to sepsis is suspected but not yet confirmed, antibiotics are typically started out of an abundance of caution or to meet CMS-imposed hospital quality measures.7 Once antibiotics have been initiated, determining when to modify, de-escalate, or safely stop therapy can be equally challenging and clinicians often assume a “one size fits all” approach based on guidelines dictating standard duration by condition or pathogen.
The importance of antibiotic stewardship
While rapid response to serious infection and sepsis is paramount, antibiotic administration can cause harm to patients and society at large. Antibiotic overuse contributes to selective pressure resulting in multi-antibiotic resistant organisms. In its 2013 report on antibiotic resistance threats, the Centers for Disease Control and Prevention (CDC) noted that antibiotic-resistant infections aff lict over 2 million people and cause approximately 23,000 deaths annually. Adverse effects associated with prolonged and sometimes even short duration antibiotic therapy include severe allergic reactions, end organ and neurological toxicity, localized or systemic candidiasis, and significant disruption of the microbiota throughout the body. The microbiome can take up to a year to recover, during which time intrusion and growth of harmful organisms such as Clostridioides difficile (C. diff) can occur.8-10 Tamma et al. found that 20 to 30 percent of antibiotic days received by hospitalized patients are unnecessary and account for 20 percent of all antibiotic adverse events, while Shehab et al. determined that such events were responsible for 19 percent of all emergency department (ED) visits.8,11
Procalcitonin can aid therapeutic decision-making that supports antibiotic stewardship
In 2015, CDC released guidance on the core elements of antibiotic stewardship. This guidance emphasizes the importance of developing a hospital-wide program based on a multidisciplinary team approach that includes doctors, nurses, pharmacists, administration, and laboratorians.12 At the time of its introduction, CDC commented on the evolving role played by diagnostic tests, and noted that procalcitonin (PCT)—an early marker of sepsis and severe bacterial infection—has been successfully incorporated into stewardship programs.
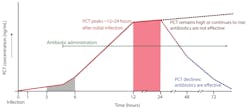
PCT is a prohormone. Under normal conditions, it is produced only by the thyroid C-cells and processed to yield calcitonin and katacalcin. During severe localized or systemic infections, however, endotoxin stimulates PCT release from adherent monocytes, which induce further production by adipocytes in nearly every tissue type (especially the liver). PCT elevates within three to six hours of infection, peaks within approximately 12 to 48 hours, and has a half-life of approximately 20 to 35 hours. PCT kinetics and the impact of antibiotic efficacy are displayed in Figure 1.13 Decision-making thresholds for initiating and stopping antibiotic therapy are presented in Tables 1 and 2.14
To date, the majority of studies have focused on two specific uses: Guidance on de-escalating or stopping therapy in patients diagnosed with sepsis, and guidance on starting and de-escalating or stopping antibiotic therapy for patients with lower respiratory tract infections (LRTI).
Figure 1. PCT kinetics (adapted from Meisner).13
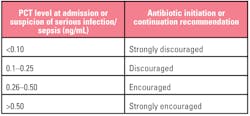
Table 1. Interpreting PCT results for initiating antibiotic therapy. Patient presentation and clinical judgement should always play a significant role in decision-making however, especially since PCT can be elevated under some conditions not associated with infection, therapy should not be administered on the basis of test results in isolation.14
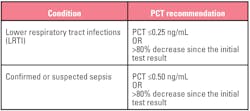
Table 2. Suggested values for discontinuing antibiotic therapy.
Patient and financial benefits of PCT guided-therapy for sepsis
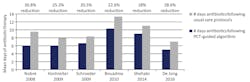
Various studies have demonstrated that serial PCT values can safely help guide de-escalation and cessation of antibiotic therapy, resulting in an 18 to 37 percent reduction in antibiotic duration. This variability is most likely related to the cut-off used for stopping antibiotics (one study specified not stopping therapy until PCT reached 0.1 ng/mL) and the degree of clinician adherence to the algorithm (between 44 and 71 percent) Figure 2.15-20 Economic modeling conducted by Mewes et al. projected that PCT guidance could reduce total per patient costs by over $11,300 when considering aggregate costs associated with hospital length of stay, days of mechanical ventilation, laboratory, antibiotics, productivity loss, and development of antibiotic resistance and C. diff infections. They also estimated that in the U.S. alone, PCT guidance would result in development of 13,222 fewer cases of antibiotic resistance and 16,103 fewer C. diff infections.21
Figure 2. Reduction in days of antibiotic therapy in patients with sepsis using PCT guidance.15-20
PCT guided-therapy in LRTI
As with sepsis, Mewes et al. projected that using PCT guidance in the treatment of LRTI could result in a cost savings of $2,867/patient, development of antibiotic resistance in 64,466 fewer patients, and 31,487 fewer cases of C. diff.21 Several studies have demonstrated safe reduction of mean antibiotic duration for patients with LRTI through a combination of withholding inappropriate antibiotic therapy and reduced therapy days. The ProHOSP (Procalcitonin-guided antibiotic therapy and hospitalization in patients with lower respiratory tract infections) study conducted in six academic and nonacademic Swiss hospitals was one of the earliest and most rigorous trials. The study evaluated the impact of applying the Table 1 decision cut points and therapy recommendations to the care of 1,361 adults (≥18 years) who presented to EDs with community acquired pneumonia (CAP), bronchitis, acute exacerbation of COPD (aeCOPD), or other suspected LRTI.22 PCT was tested at presentation and electronically reported within approximately one hour to physicians, along with treatment recommendation. If antibiotics were withheld based on the PCT result, PCT was retested within six to 24 hours. If hospitalization was required, PCT was retested on days three, five, and seven to determine the need for modifying, de-escalating, or stopping antibiotic therapy. Using this algorithm, mean antibiotic exposure decreased from 10.7 days to 7.2 days per patient, representing a 32 to 65 percent reduction, depending on the patient’s final diagnosis. Similarly, the rate of total antibiotics prescribed decreased by eight to 27 percent, and the antibiotic adverse event rate decreased from 33 to 23 percent. Most of the reductions in exposure resulted from avoiding inappropriate antibiotic administration for bronchitis and nonbacterial aeCOPD with no increase in adverse outcomes within 30 days (death, ICU admission, complications, or reinfection).
Several other studies conducted using guidance algorithms and protocols that were the same or similar to the ProHOSP study have reported variable results, ranging from 0 to 47 percent reduction in overall antibiotic days of treatment.22-28 Chief among the negative studies was the recently reported ProACT (Procalcitonin Antibiotic Consensus Trial), which reported no reduction in antibiotic usage.26,29 A 2015 study by Branche et al. also found no difference in overall antibiotic exposure using PCT guidance, although a trend was noted toward fewer days of prescribed antibiotics, and significantly fewer patients were still receiving antibiotics at discharge.24
This has engendered some controversy, however close comparison of several of these studies provides some insight into design and execution differences between trials in which antibiotic usage was successfully reduced and in those where no benefit was found. These observations could help hospitals develop and implement their own successful PCT practices.
Table 3. Patient diagnosis in PCT cohort by percentage of study/population.
1. Algorithm compliance among clinicians was greatest in the studies demonstrating significant reductions in antibiotic days of therapy. Adherence to the ProHOSP study protocol was 90 percent. An evaluation of pre- versus post-PCT implementation on existing stewardship practices at a small rural U.S. hospital with 92 percent adherence to protocol demonstrated a 47 percent reduction in total days of therapy (DOT).25 Adherence in studies reporting up to 25 percent reduction in antibiotic DOT was 70 to 81 percent, with the exception of one study where adherence was only 59 percent.23,27,28 In comparison, although adherence was ~74 percent in the ED in the ProACT study, overall adherence over the course of the hospital stay was less than 64 percent in both the ProACT and Branche, et al. studies.24,26 In addition, whereas most protocols specified explicit algorithm overrule criteria, the ProACT study physicians were allowed complete autonomy over antibiotic usage and chose not to enroll patients who had conditions for which clinicians are unlikely to withhold antibiotics for any reason. In their analysis, Albrich et al. determined hospitals with higher adherence saw the greatest reductions in directly observed therapy (DOT).23
2. Reduction in antibiotic use can be affected by the study population and whether or not results are actionable. The majority of patients enrolled in studies supporting PCT-guided antibiotic reductions were diagnosed with community-acquired pneumonia (CAP) and were generally sicker overall than in either the Branche or ProACT studies (Table 3). This has lead Townsend et al. and others to suggest that one of the reasons no difference was observed between cohorts was because these studies were heavily weighted toward low acuity patients who are less likely to be prescribed antibiotics or are typically prescribed antibiotics for shorter duration.28 Furthermore, as pointed out by UC San Diego’s Dr. G. Seymann in a recent industry-sponsored webinar, PCT results are most useful when the results are actionable. Measuring PCT might not be useful in every situation. For example, if symptoms clearly indicate CAP, a single PCT value may not provide additional benefit at the time of diagnosis. However, serial measurements can help the clinician determine if antibiotics are effective before culture results are available and when it is safe to stop treatment. Likewise, if bacterial etiology is uncertain, PCT can help determine if antibiotics are called for, but if LRTI is clearly viral (e.g., positive influenza PCR), PCT might not provide additional useful information.
3. Antibiotic decision-making is best supported by timely and efficient availability of results. Results were available within one hour in the ProHOSP and Broyle’s studies. In ProHosp, results were provided directly to care providers via the study website along with treatment recommendations. In the Broyle’s study, the PCT order was made via prechecked field on the admission order set if infection was suspected: Results were built into the laboratory report electronically and could be accessed by mousing over the test. In contrast, mean turnaround time (TAT) was 1.5 to 1.6 days in studies reporting only 20 to 25 percent reduction. The authors of these studies commented that DOT reductions likely would have been greater had TAT been much shorter. Both of these studies conducted PCT testing in batch once a day during the week, and one study did not provide PCT testing on weekends. This highlights the benefit of running PCT on analyzers capable of handling random access and stat orders. While some facilities have found one manufacturer’s point-of-care PCT test useful for achieving rapid results, it should be emphasized that this test is not sensitive enough to be used for stopping antibiotics when conducting serial testing. Only tests based on the B.R.A.H.M.S. PCT assay currently offer this level of sensitivity.
4. Staff training and continuing education at all levels can contribute to successful program implementation. Studies describing reduced DOT incorporated several elements of formal, in-person training on the rationale behind PCT guidance and how to use the algorithm effectively. Training was provided in the form of seminars and in-service education to all staff across multiple departments who would be in a position to prescribe antibiotics, including residents, nurse practitioners, and physician’s assistants. Training was also provided to the lab and pharmacy personnel in some cases. In one study, both online and in-person training was made available. Training and support was ongoing in some cases, and additional supporting materials were made available in the form of posters, pocket cards, handouts on trial and algorithm details, and embedded in laboratory results. Studies with the highest participation in training noted the highest adherence to protocol and the greatest reduction in DOT.
5. Having a dedicated coordinator or PCT champion contributes to program success. The Broyles study illustrates the value of having one department oversee and champion PCT guidance, which in this case was the pharmacy. This decision makes credible sense as the pharmacists and staff were already responsible for oversight of their antibiotic stewardship program. Consequently, pharmacists created opportunities to mentor staff, conduct continuing education, and even override physician decisions to not order PCT testing or contravene algorithm guidance when infection or sepsis was suspected. As a result, this institution realized a 47 percent decrease in antibiotic DOT above and beyond reductions already achieved through their existing stewardship program. Other sites have successfully cultivated infectious disease specialists, nurses, and multidisciplinary teams made up of clinicians, pathologists, and laboratorians to fill these roles.
PCT limitations
Although PCT is highly specific for bacterial infection, there are some situations in which PCT can give a false-positive result if not used with discretion. PCT clearance appears to be affected by renal disease and can be elevated in late-stage chronic kidney disease patients regardless of dialysis requirement in the absence of infection.30 PCT can also elevate in the case of severe inflammation, as might occur with significant surgery, polytrauma, severe pancreatitis or liver damage, severe burns, medullary thyroid cancer, small cell lung carcinoma, prolonged cardiogenic shock, and in response to some cytokine-stimulating medications.13,31 PCT can also elevate with some fungal and malarial infections, however, Miglietta et al. note that PCT can distinguish between bacterial sepsis and systemic candidiasis.31
Conclusion
PCT can be a useful addition to the clinician’s armamentarium. When used according to practices exemplified by several studies, PCT can support antibiotic decision-making essential for reducing unnecessary antimicrobial therapy contributing to the development of antibiotic-resistant organisms and short- and long-term adverse events.
Useful Webinars
Procalcitonin 2019: Potential and Pitfalls. https://www.youtube.com/watch?v=9dWW7oQVk3A
Implementing Procalcitonin: A Team Approach. https://www.youtube.com/watch?time_continue=6&v=6gNh-w_ktTc
The Role of Procalcitonin in Bacterial Infection and Patient Management: A Pharmacy Perspective. https://www.youtube.com/watch?v=gjAYeJIv4A0
Procalcitonin: Once is Not Enough. The Critical Role of Serial Testing. https://www.youtube.com/watch?v=cWWB7e_S_Ws
The Importance of Procalcitonin for Antimicrobial Stewardship in Patients with Lower Respiratory Tract Infections in the Emergency Department. https://www.labroots.com/ms/webinar/importance-procalcitonin-antimicrobial-stewardship-patients-lower-respiratory-tract-infections-emerg
REFERENCES
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45:486-552.
- Spotlight: Overdiagnosis and Delay: Challenges in Sepsis Diagnosis. Agency for Healthcare Research and Quality: Patient Safety Network, 2018. Accessed May 21, 2019, at https://psnet.ahrq.gov/webmm/case/458/Spotlight-Overdiagnosis-and-Delay-Challenges-in-Sepsis-Diagnosis.
- Clifford KM, Dy-Boarman EA, Haase KK, et al. Challenges with Diagnosing and Managing Sepsis in Older Adults. Expert Rev Anti Infect Ther. 2016;14:231-41.
- Gupta S, Sakhuja A, Kumar G, et al. Culture-Negative Severe Sepsis: Nationwide Trends and Outcomes. Chest 2016;150:1251-9.
- Campbell SG, Marrie TJ, Anstey R, et al. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest 2003;123:1142-50.
- Vincent JL. The Clinical Challenge of Sepsis Identification and Monitoring. PLoS Med. 2016;13:e1002022.
- Barbash IJ, Rak KJ, Kuza CC, Kahn JM. Hospital Perceptions of Medicare’s Sepsis Quality Reporting Initiative. J Hosp Med. 2017;12:963-8.
- Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of Adverse Events With Antibiotic Use in Hospitalized Patients. JAMA Intern Med. 2017;177:1308-15.
- Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124:4212-8.
- Rashid MU, Zaura E, Buijs MJ, et al. Determining the Long-term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin Infect Dis. 2015;60 Suppl 2:S77-84.
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-43.
- Core Elements of Hospital Antibiotic Stewardship Programs. CDC. 2015. Accessed July 5, 2019, at https://www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html.
- Meisner M. Procalcitonin - Biochemistry and Clinical Diagnosis. 1st ed. Bremen - London - Boston: UNI_MED Verlag AG; 2010:128.
- Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171:1322-31.
- Bouadma L, Luyt C-E, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. The Lancet. 2010;375:463-74.
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. The Lancet Infectious Diseases. 2016;16:819-27.
- Hochreiter M, Kohler T, Schweiger AM, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13:R83.
- Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498-505.
- Schroeder S, Hochreiter M, Koehler T, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394:221-6.
- Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med. 2014;190:1102-10.
- Mewes J, Pulia M, Mansour M, et al. PCT-guided antibiotic stewardship versus usual care for hospitalised patients with suspected sepsis or lower respiratory tract infections in the US: a model-based cost analysis. AACC. Chicago, IL: Panaxea; 2018.
- Schuetz P, Christ-Crain M, Thomann R, et al. Effect of Procalcitonin-Based Guidelines vs Standard Guidelines on Antibiotic Use in Lower Respiratory Tract Infections. JAMA. 2009;302.
- Albrich WC, Dusemund F, Bucher B, et al. Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in “real life”: an international, multicenter poststudy survey (ProREAL). Arch Intern Med. 2012;172:715-22.
- Branche AR, Walsh EE, Vargas R, et al. Serum Procalcitonin Measurement and Viral Testing to Guide Antibiotic Use for Respiratory Infections in Hospitalized Adults: A Randomized Controlled Trial. J Infect Dis. 2015;212:1692-700.
- Broyles MR. Impact of Procalcitonin-Guided Antibiotic Management on Antibiotic Exposure and Outcomes: Real-world Evidence. Open Forum Infect Dis. 2017;4:10.1093/ofid/ofx213.
- Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N Engl J Med. 2018;379:236-49.
- Kristoffersen KB, Sogaard OS, Wejse C, et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission--a randomized trial. Clin Microbiol Infect. 2009;15:481-7.
- Townsend J, Adams-Sommer V, Galiatsatos P, et al. Procalcitonin-guided antibiotic therapy for lower respiratory tract infections in a US academic medical center. ID Week. San Francisco, CA 2018.
- Huang DT, Angus DC, Chang CH, et al. Design and rationale of the Procalcitonin Antibiotic Consensus Trial (ProACT), a multicenter randomized trial of procalcitonin antibiotic guidance in lower respiratory tract infection. BMC Emerg Med. 2017;17:25.
- Grace E, Turner RM. Use of procalcitonin in patients with various degrees of chronic kidney disease including renal replacement therapy. Clin Infect Dis. 2014;59:1761-7.
- Miglietta F, Faneschi ML, Lobreglio G, et al. Procalcitonin, C-reactive protein and serum lactate dehydrogenase in the diagnosis of bacterial sepsis, SIRS and systemic candidiasis. Infez Med. 2015;23:230-7.
About the Author

H. Roma Levy, MS
holds an MS from UC Santa Cruz in molecular biology with an emphasis in chronobiology, in which she conducted independent research. As a medical writer for Siemens Healthineers, Ms. Levy has written or co-authored multiple articles and clinical educational presentations over the last 16 years in diverse areas, including immunology and infectious disease, endocrinology, cardiology, and opioid addiction.

Monet Sayegh, MD, MA, BS, MT (ASCP) SH, CLS
Is a Senior Medical/Clinical Consultant and a member of the Disease State Education group at Siemens Healthineers with focus on cardiovascular, oncology, and infectious diseases.