A practical approach to evaluating requests in testing new antimicrobials
Many new antimicrobials have recently come to market to address the rising threat of multidrug-resistant (MDR) bacteria. Clinical and public health laboratories are critical participants in the management of patients with MDR infections and must develop a plan for antimicrobial susceptibility testing (AST) of these new agents, either in-house or at a reference laboratory.
Herein, we present an example of a practical approach to this challenge. Let’s consider how a laboratory might respond to the scenario of receiving the following request from the institutional antimicrobial stewardship team:
Can the lab start testing carbapenem-resistant Enterobacteriaceae (CRE) for ceftazidime-avibactam (CZA; AVYCAZ) susceptibility?
Consider the clinical need
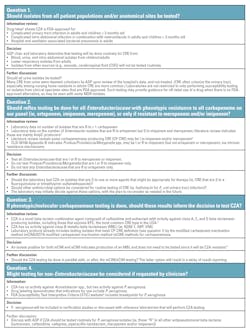
The antimicrobial stewardship program (ASP) chair has requested all CRE be tested for CZA susceptibility. This request seems simple, but careful consideration should be given to different testing approaches. Some key questions for the laboratory to consider are listed in Table 1.
Testing in-house vs send-out?
This requires consideration of:
- Expected volume of testing, based on the number of carbapenem-resistant Enterobacteriaceae from last year’s antibiogram.
- Availability of testing options and materials.
- Capacity to perform testing in-house.
- Staffing capacity to perform verification studies, write standard operating procedures (SOPs), etc.
- Impact of testing option(s) on turnaround time to results.
A basic cost-analysis of in-house vs send-out testing should be performed, keeping in mind that the results of testing CZA are often critical to patient care.
Close collaboration with infectious diseases, critical care physicians, laboratory medicine, and administrative colleagues is essential to ensure an optimal workflow.
For the purpose of this exercise, let’s assume the laboratory encounters ~100 isolates per year that meet the testing criteria defined in Table 1, and determines testing should be done in-house.
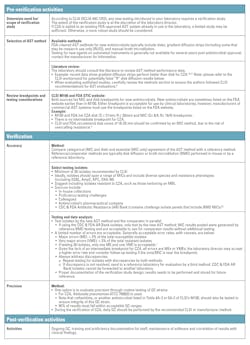
How to perform the verification studies
The essential components of a verification study are presented in Table 2; further guidance is found in references.6-9
How to perform QC? Should we consider an IQCP?
CLIA requires a laboratory to either perform AST QC daily or develop an individualized quality control plan (IQCP).4 In many laboratories, CRE are relatively uncommon and CZA will be tested infrequently (i.e., less than once a week). As such, the lab may choose to not perform an IQCP, but to perform QC each time a patient isolate is tested.
What results should I expect? (i.e., how often are carbapenem-resistant isolates R to CZA?)
In vitro susceptibility rates of Enterobacteriaceae to CZA are very high, with > 99.5 percent susceptibility overall, and > 97 percent among class A and D carbapenemase-producing isolates.13,14
In contrast, as highlighted previously, little to no activity is expected against MBL-producing Enterobacteriaceae. If the laboratory encounters a high rate of resistance, this should be investigated to ensure it is not due to technical error.
Summary
In summary, broad-spectrum agents such as ceftazidime-avibactam have strongly enhanced the therapeutic arsenal against MDR gram-negative organisms, an important cause of morbidity and mortality in patients in hospitals and long-term care facility settings.
Implementing testing and completing a verification study for a new antimicrobial agent such as CZA carry additional workload which may seem daunting to the laboratory. However, accurate and timely microbiological testing is instrumental in identifying effective therapies that may significantly impact patient outcomes and enable effective resource allocation.
REFERENCES
- FDA. Access data. 2018; https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206494s004lbl.pdf. Accessed March 18, 2019.
- Yang FC, Yan JJ, Hung KH, Wu JJ. Characterization of ertapenem-resistant Enterobacter cloacae in a Taiwanese university hospital. J Clin Microbiol. 2012;50(2):223-226.
- Karlowsky JA, Lob SH, Kazmierczak KM, et al. In vitro activity of imipenem against carbapenemase-positive Enterobacteriaceae isolates collected by the SMART global surveillance program from 2008 to 2014. J Clin Microbiol. 2017;55(6):1638-1649.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. CLSI Supplement M100. Wayne, PA: CLSI; 2019.
- FDA. Antibacterial Susceptibility Test Interpretive Criteria. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm575163.htm. Accessed April 6, 2019.
- Sharp SE CR. Cumitech 31A: Verification and Validation of Procedures in the Clinical Microbiology Laboratory. ASM Press. 2009.
- CLSI. Verification of Commercial Microbial Identification and Antimicrobial Susceptibility Testing Systems. 1st ed. CLSI M52. Wayne, PA: Clinical and Laboratory Standards Institute; 2015.
- Jorgensen JH. Putting the new CLSI cephalosporin and carbapenem breakpoint changes into practice in clinical microbiology laboratories. J Pediatric Infect Dis Soc. 2012;1(2):169-170.
- Humphries RM, Ambler J, Mitchell SL, et al. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol. 2018;56(4): pii: e01934-17.
- Shields RK, Clancy CJ, Pasculle AW, et al. Verification of ceftazidime-avibactam and ceftolozane-tazobactam susceptibility testing methods against carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol. 2018;56(2): pii: e01093-17.
- Wenzler E, Lee M, Wu TJ, et al. Performance of ceftazidime/avibactam susceptibility testing methods against clinically relevant gram-negative organisms. J Antimicrob Chemother. 2019;74(3):633-638.
- CDC & FDA AR Isolate Bank. 2018; https://www.cdc.gov/drugresistance/resistance-bank/index.html. Accessed April 6, 2019.
- Sader HS, Castanheira M, Flamm RK, et al. Ceftazidime/avibactam tested against gram-negative bacteria from ICU and non-ICU patients, including those with ventilator-associated pneumonia. Int J Antimicrob Agents. 2015;46(1):53-59.
- de Jonge BL, Karlowsky JA, Kazmierczak KM, et al. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM global surveillance study (2012 to 2014). Antimicrob Agents Chemother. 2016;60(5):3163-3169.
About the Author

Catherine A. Hogan
completed her infectious diseases fellowship at McGill University, followed by a medical microbiology fellowship at Stanford University. Prior, she completed a MSc in epidemiology at the London School of Hygiene and Tropical Medicine. Catherine is currently a visiting instructor and global health diagnostics fellow at Stanford where she conducts research on innovative diagnostic methods, TB diagnostics, and assessing the clinical impact of microbiological testing.