Since SARS-CoV-2 began spreading around the world in 2020, companies have developed a range of products to address large-scale diagnostic- and surveillance-testing needs.
Rapid RT PCR test
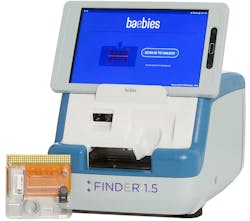
The rapid RT-PCR test for SARS-CoV-2 features a disposable cartridge with all reagents included, providing results in 17 minutes or less. The test runs on the FINDER 1.5, which is a toaster-sized instrument powered by digital microfluidics, without pumps, tubing, or valves.
Baebies
Run Controls
The SARS-CoV-2, Flu, RSV Positive and Negative Run Controls are unassayed external controls used to monitor the presence or absence of SARS-CoV-2, Influenza A, Influenza B and RSV (A). The products are formulated in a synthetic matrix and contain human genomic DNA.
Bio-Rad Laboratories
Molecular diagnostics
The Aptima SARS-CoV-2 assay is a molecular diagnostic test that utilizes proprietary TMA (Transcription-Mediated Amplification) technology to detect RNA from SARS-CoV-2. Meanwhile, the Panther Fusion SARS-CoV-2 real-time PCR assay also detects RNA from SARS-CoV-2. Both tests process 1,000 tests in 24 hours and deliver results in 3.5 hours or less.
Hologic
Quality tool
AccuPlex SARS-CoV-2 in synthetic oral fluid is a research tool for developers creating saliva-based SARS-CoV-2 assays, as well as a complete quality solution for clinical laboratories employing such tests. The product includes positive vials containing the full SARS-CoV-2 viral genome, and human RNase P sequences to monitor sample collection.
SeraCare
RT-PCR test
The QIAstat-Dx Analyzer, combined with QIAstat-Dx assay cartridges, uses real-time RT-PCR to detect multiple respiratory pathogens in about an hour. The QIAstat-Dx Analyzer and cartridges are designed as a closed system that contains on board all necessary reagents, enabling an easy-to-use workflow.
Qiagen
Third party control
The Acusera SARS-CoV-2 Antibody Control comprises both reactive and non-reactive controls for Anti SARS-CoV-2, supporting assay validation and routine performance monitoring of serological assays for COVID-19. The control, which is CE marked, is supplied in a liquid format with a 30-day open vial stability.
Randox
Variant detection
The high-throughput Allplex 2019-nCoV Assay (FDA EUA) detects 3 different target genes (E, RdRP, and N) of SARS-CoV-2 and covers multiple variants in a single reaction tube. The test provides results within 1 hour and 50 minutes. It is designed for maximum throughput to accommodate high volume testing.
Seegene Technologies
Incubator
The Heratherm Advanced Protocol Security Microbiological Incubator enables reliable heat virus inactivation to protect medical laboratory personnel from infection and allow safe sample handling. It offers temperature uniformity, continuous operation, and a sterile environment for sample protection and reliable results.
Thermo Fisher Scientific
Foam swab
The specialty swab includes an applicator tipped with 100ppi medical grade polyurethane foam with high particle collection capacity and sturdy handle for control. Used widely for COVID-19 testing, the swab also can be used for various medical, diagnostic, and DNA testing applications.
Puritan Medical Products
Automated PCR test
The cobas SARS-CoV-2 Test is intended for the qualitative detection of SARS-CoV-2 in nasal, nasopharyngeal and oropharyngeal swab samples from patients with suspected COVID-19 infection. Laboratories can run the PCR test on the automated, high-throughput cobas 6800/8800 systems, which provide results in about three hours.
Roche Diagnostics
About the Author
Sign up for our eNewsletters
Get the latest news and updates