The practice of laboratory medicine began 6,000 years ago with the analysis of urine as the primary diagnostic tool available to ancient physicians. Often referred to as the “divine fluid,” urine was considered the golden liquid window through which they could glean information on mysterious inner workings of the human body. While often prone to fallacies and misinformation, many of the ancient inferences were indeed accurate. Early Hindu physicians correlated sweet tasting urine with the characteristic disease-state symptoms of diabetes mellitus. The recognition that black ants were attracted to this urine was probably the first diagnostic test in medical history. In the 4th century BCE, Hippocrates accurately described that urine was a filtrate of the humors (filtration of blood through the kidneys), that bubbles on the surface of freshly voided urine indicated long-term kidney disease (a physical symptom of proteinuria), and that an increase of urine sediment correlated with a worsening fever (leukocytes and bacteria from a urinary tract infection).1
No Simple Solution
In the most basic terms, urine is mostly water with some nitrogenous compounds, electrolytes and metabolic waste components. This generalization, however, dramatically underemphasizes the true complexity of urine and its importance as a noninvasive tool to monitor homeostasis and a myriad of different disease states. It is, quite literally, a veritable fountain of valuable diagnostic information.
Urine is an ultrafiltrate of blood plasma, representing the principal route of waste removal of products of metabolism from the body. Blood is constantly being filtered by the kidneys, receiving about a quarter of the total cardiac output. Over the course of a given day, the kidneys filter a staggering 180 liters of filtered plasma into a final urine volume of about 1.2 liters.2 The composition of urine is arguably as complex as the blood from which it is derived, however, the concentrations of those compounds are often substantially different from one another.
For example, serum creatinine, a byproduct of muscle metabolism, is tightly regulated by the kidneys. Normal serum levels are about 0.9-1.3 mg/dL in adult males and 0.6 – 1.1 mg/dL in adult females. The creatinine concentration of a random urine sample, however, can range from 40 to 300 mg/dL.3 The hyper-concentration of creatinine in the urine makes sense considering that it’s the pathway of excretion of this nitrogenous metabolic byproduct. The physiology of creatinine makes it a very useful and convenient endogenous substance to assay when evaluating for kidney function. A decrease of urine creatinine levels coupled with an increase of serum levels provides a strong indication of declining kidney function.
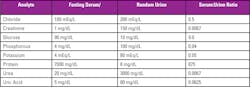
Conversely, metabolically useful compounds such as glucose, amino acids and inorganic phosphate are initially part of the tubular fluid (pre-urine) ultrafiltrate, but are mostly reclaimed back into the blood by the tubular reabsorption process. Table 1 below compares typical fasting serum versus random urine analyte concentrations from healthy individuals.4
Urine is a bewilderingly complex and constantly variable biofluid. The Urine Metabolome Database5 (http://www.urinemetabolome.ca) currently lists 4276 small molecule metabolites that can be found in human urine using current assay technologies, and this list is constantly expanding. Urine contains metabolic byproducts of all the food, drink, vitamins, drugs, environmental agents and contaminants that enter our system. It also includes byproducts from the metabolism of the trillions of microorganisms that cohabitate our bodies.
Urine + Analysis
Physical examination has a long and storied history wrought with erroneous inferences; however, it is still used today to provide important information. The most important aspects include the evaluation of color, foam, clarity, odor and concentration via physical specific gravity techniques.
Chemical examination mostly commonly employs the use of urinalysis dipsticks that contain various reagent pads to semi-quantitatively test for the presence of ascorbic acid, bilirubin, blood, creatinine, glucose, ketones, leukocytes, microalbumin, nitrite, pH, protein, specific gravity and urobilinogen. General chemistry analyzers can more precisely and quantitatively evaluate urine for analytes such as amylase, calcium, chloride, creatinine, glucose, magnesium, osmolality, phosphorous, potassium, sodium, protein, blood urea nitrogen, urea and uric acid, to name a few. As per the current status of the urine metabolome project, these commonly tested analytes only scratch the surface of the totality of possible chemical examinations.
Microscopic examination of urine sediment traditionally involves the centrifugation, concentration and slide preparation of the sample to identify the presence of a variety of formed elements via manual microscopy. These include RBCs (erythrocytes), WBCs (leukocytes), Epithelial cells (renal, transitional, or squamous), Casts (hyaline or various pathological sub-types), Crystals (various), Mucous, Bacteria, Yeast, Trichomonas, Lipids, Sperm, etc. While manual microscopy techniques are still widely utilized, the emergence of automated urine sediment analyzers, using either flow cytometry or digital imaging methodologies, are rapidly being adopted by clinical laboratories. These systems do not require centrifugation, or other special processing, leading to an improved workflow. They serve to decrease the number of samples that require more labor-intensive and time-consuming confirmatory manual microscopy and allow for process standardization. Another major advantage with automated urine sediment analysis is that more patient samples can be screened, including those that are negative by reagent strip analysis. Using traditional laboratory algorithms, these samples would not have been examined further, possibly missing pathologic samples. A study from the University Hospital Zurich found that the combination of dipstick and automated urine sediment analysis using the Iris iQ200 and Sysmex UF-100 increased the sensitivity of screening to about 98 percent.6
Quantimetrix offers an excellent free mobile app called Urinalysis Made Simple that serves as a convenient urinalysis reference tool for laboratory professionals, teachers and students.
The Right Urine for the Job
The analysis of urine for disease characterization and monitoring is favored by physicians due to convenience and non-invasiveness. Due to the inherent hour-by-hour variation in the composition of urine , it’s critical to collect the most appropriate specimen type, volume, and handling for the analyses to be performed. Not every urine sample is fit for the purpose of every type of test.
The three basic urine specimen types are random, first void (or first morning) and timed samples.
Random urine analysis is the most frequently performed for routine screening as it is the most convenient specimen to obtain. It can be collected anytime and does not require special patient preparation or instruction. These samples are the most affected by changes in fluid intake and exercise so they may not be the best refection of a patient’s condition but are typically satisfactory for routine screening. Typically, random urine samples should be midstream (or clean catch) which is collected after the urine flow has started, helping to prevent contamination from bacteria and epithelial cells.
First void urine, as the name implies, requires that the patient voids before going to bed then collects a specimen first thing in the morning. While not the most convenient specimen to obtain, this urine has been retained in the bladder for about eight hours and is ideal for cytology analysis of RBCs, WBCs and epithelial cells. It’s also ideal for analytes that require concentration or incubation for detection, such as casts, nitrites and protein.2
Timed urine samples are collected over a specified time period to help normalize for the variability of urine composition. These are typically collected over a continuous eight- to 24-hour period, and are particularly well suited for quantitative determinations for analytes such as creatinine, albumin, urea nitrogen, glucose, sodium, potassium, etc. Strict adherence to the timing and collection protocol is critical to obtaining useful results. A preservative may be necessary for timed urine to maintain sample integrity. Refrigeration is a common preservation method but may result in the precipitation of crystals. A variety of chemical preservatives may be used but they are not always suitable for all testing needs. There are also several commercially available urine transport tubes that contain preservative cocktails that are well suited for certain tests.2
Golden Future
Laboratory medicine’s oldest practice continues to be relevant to this day. Urine is easily obtained and will continue to provide valuable information that is not available from any other source. Advances in urinalysis automation will certainly improve sensitivity, specificity and standardization. The recent push toward fully automated systems, such as the new Siemens Atellica 1500, will set the new standard for accuracy and efficiency in the clinical laboratory. The vast number of metabolites being compiled by the Human Urine Metabolome database project will undoubtedly reveal new biomarkers of disease and exciting new breakthroughs for this divine fluid.
REFERENCES
- Armstrong JA, Urinalysis in Western culture: A brief history, Kidney International. 2007: Mar; 71(5): 384-387.
- Brunzel N, Fundamentals of Urine & Body Fluid Analysis. 3rd Ed. St. Louis: Saunders; 2012.
- Pagana K, Pagana TJ eds. Mosby’s Manual of Diagnostic and Laboratory Tests. 5th Ed. St. Louis, Missouri. 2014.
- Free AH, Free HM, Urinalysis in Clinical Laboratory Practice. Reissue, Boca Raton: CRC Press; 1975.
- Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, et al. (2013) The Human Urine Metabolome. PLoS ONE 8(9): e73076. doi:10.1371/journal.pone.0073076.
- Shayanfar N, Tobler, U, von Eckardstein A, Bestmann L, Automated urinaylsis: first experiences and a comparison between the Iris iQ200 urine microscopy system, the Systmex UF-100 flow cytometer and manual microscopic particle counting. Clin Chem Lab Med. 2007: 45(11): 1570.
About the Author

Brian Fernandez
serves as Director of Research and Development for Redondo Beach, California-based Quantimetrix.