Urine-specific gravity: Specific clinical relevance
The goal of urine is to eliminate urea and other byproducts from the body. Specific gravity, also known as relative density, is defined as finding the ratio between the density of substances versus the density of solution. This is distinguishable from the precision of osmolarity as osmolarity defines an exact number based on the particles within a density and is not subject to change based on weights.1 For laboratories, urinary analysis (uranalysis) uses urine-specific gravity (USG) as a timely estimate for finding the concentration of byproducts within a patient's urine. Urine-specific gravity is the ratio of the density of urine to the density of water (Figure 1). When interpreting results for uranalysis, USG provides information on a kidney’s ability to filter, the status of hydration in patients, and identifies plausible long-term health conditions such as diabetes insipidus or loss of renal tubular abilities.
The base of any urine sample is the discarded water from a patient’s body containing waste urea, traces of various hormones, vitamins, drug metabolites, and other organic and inorganic solutes. Aside from urea, the organic components in a sample may include creatinine, uric acid, hippuric acid, urobilin, and other carbon-based byproducts. The inorganic components in urine samples include, but are not limited to, sodium chloride, potassium, sulfate, phosphate, ammonium, magnesium, and calcium.2
Kidneys selectively reabsorb chemicals, minerals, and water through glomerular filtration. This process is conducted individually by roughly one million nephrons per kidney, which filters blood through a cluster of branching red blood vessels called the glomerulus. The nephrons return necessary substances including salt, glucose, and water, back to the blood while removing waste through the ureter. The amount of waste excreted in urine is determined by the osmotic gradient in the medulla; the water is regulated by the naturally occurring antidiuretic hormone (ADH), which is regulated by the renin-angiotensin-aldosterone system that controls the flow of blood to and within the kidney.3,4 ADH accomplishes regulation by binding to the receptors on principal cells. Principal cells account for two-thirds of the cell type in the initial collecting tubule of kidneys, whereas intercalated cells account for the other third.5 When ADH attaches itself onto the receptors, the integral membrane proteins, aquaporins, change shape allowing for water to pass through. The water is reabsorbed through the renal collecting tubule due to the higher concentration gradient in the medullary interstitium.3,4 When ADH levels become high, tubular walls become more permeable; when ADH levels are low, the impermeable tubular walls retain water leading to diluted urine.
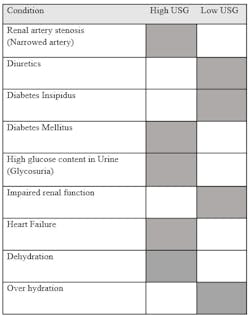
A kidney’s reabsorption ability is one of the first renal functions impaired for diabetic patients. In addition, urinary tract issues and inflammation of different areas of the kidneys will also lead to impairment.6 The ratio at which substances are excreted notifies healthcare professionals of hydration, plausible kidney problems, and other underlying conditions. Risk factors affecting kidneys can be separated into two categories — controllable and non-controllable. Non-controllable factors are predetermined and cannot change in an individual such as age, race, sex, and genetic history. However, among the controllable factors are hydration, diet, and medicine, which affect the concentration of solutes in urine.
Factors affecting urinary-specific gravity
Hydration is the largest controllable factor to impact USG. Higher water intake correlates to lower USG due to a higher volume of discarded water to solutes in urine; inversely, lower hydration raises USG due to a higher concentration of solutes in a lower urine volume.2 Depending on a patient’s diet, the contents may alter USG. In a 2023 study, the biomarkers USG and creatinine-adjustments were analyzed.7 This study showed that while creatinine-adjustments in serum and urine both provided information of kidney function, USG could be used as a parameter for correcting hydration status in patients.7
Routine medicine is an important factor that can alter an otherwise “normal” reading. Depending on how a drug reaches the activation site will determine the impact on USG. Diuretic medications, described as “water pills,” have three different binding methods to reach their activation site and remove excess salt, water, and sometimes potassium by increasing excretion.8 Increasing the amount of water excreted in the body and removing the smaller ions lowers the USG in patients. Blood pressure medications, in particular alpha-blockers, will also increase urination. Alpha-blockers will interact with receptors resulting in smooth muscles to vasoconstrict or by blocking receptors that will cause vasodilation.9 Other types of blood pressure medications such as calcium channel blockers will yield similar effects by increasing secretion. Without increasing fluid intake, or proper maintenance, a patient can quickly become dehydrated.
When secretion increases to abnormal amounts, known as polyuria, it can reflect certain healthcare conditions. Diabetes mellitus is a condition caused by defects within the pancreatic production of insulin or the function of insulin. The result is an increase in glucose concentrations seen throughout the body floating in the liver and bloodstream. To counteract the excess floating glucose that cannot be reabsorbed or used, the body increases the excretion rate to remove the excess glucose.2 The urine excreted is highly concentrated and turbid due to glucose, but smaller in volume due to polyuria resulting in a higher USG. Diabetes insipidus is another condition that results in polyuria but is caused by the reduction of ADH or decreased response of ADH.10 The resulting urine is highly diluted due to aquaporins remaining shut and not allowing water to properly reabsorb back into the kidney and body.
Common tests for measuring USG
USG is measured through refractometry or reagent test strips.12 When the quantity of urine cannot fill a small container, refractometry provides an advantage in clinical settings. Refractometry works through a refractive index meaning the measurement of how much light bends or refracts upon entering a material.13 In clinical refractometry, a prism measures the velocity of light in the air and compares it to the velocity of light in the urine. Refractometry requires only one to two drops to cover the entirety of the prism. The concentration of dissolved particles present in the solution determines velocity and angle due to light passing through the solution. The wavelength of light used in a sample can affect the reading, however, in a laboratory setting these variables are consistent and less likely to affect readings. One of the advantages of refractometry is that it does not require specific temperature to provide accurate information; between 15℃ to 38℃ is enough to ensure accurate results.
Reagent strips are analyzed through manual hand dips or urinalysis automation such as the Clinitek system. The reagent pad of the strips utilizes bromothymol blue as the indicator. The color changes in Multistix and Chemstrip function on the dissociation constant (pKa) in alkaline mediums. The color shift changes from alkaline blue at 1.000 to acidic yellow at 1.030 as the USG rises. Higher concentrations lead to more released hydrogen ions causing acidic conditions. Based on a 24-hour urine collection, the typical range for USG in a healthy individual is from 1.015 to 1.025.2 A limitation of reagent strips is protein content; if protein reaches 100 to 750 mg/dL it may cause false elevated USG readings.
Analyzing special cases
USG is considered in other assessments of physiology. Metabolomics is the study of small molecules within a biological sample. The applications of metabolomics can be used to study the relationship between diet and the organism; in other cases, clinical evaluations of diseased states.14 In a 2019 comprehensive assessment of various samples and studies, urine specimens were analyzed for preanalytical factors and how they influenced the metabolomic states. The urine concentration produced from a single patient can change drastically from sample to sample. The assessment of urine can account for some variation by normalizing metabolite levels. The goal of normalization is to reduce the variation of nonbiological variations from biological variations. Specifically in urine, samples are normalized through creatinine, osmolarity, or specific gravity.15 The paper looked at three studies where normalization was used at three different times during a procedure and evaluated for consistency and accuracy.
USG is dependent on the size and number of particles present within a solution. When evaluating renal function, it gives a fast, approximate state of patients and possible risk factors. Determining therapies and treatments should not rely solely on USG, but when used as an approximate tool, in addition to other markers, it can help provide fast and timely treatment and assessments.
REFERENCES
1. Larkins MC, Thombare A. Osmometer. StatPearls Publishing; 2022.
2. Strasinger SK, Schaub M. Urinalysis and Body Fluids. F.A. Davis Co. Published online 2001:37-41.
3. Cuzzo B, Padala SA, Lappin SL. Physiology, Vasopressin. StatPearls Publishing; 2023.
4. Your kidneys & how they work. National Institute of Diabetes and Digestive and Kidney Diseases. Published October 16, 2023. Accessed November 15, 2023. https://www.niddk.nih.gov/health-information/kidney-disease/kidneys-how-they-work.
5. Verlander JW. Solute reabsorption. Cunningham’s Textbook of Veterinary Physiology. Published online. 2020:489-500. doi:10.1016/b978-0-323-55227-1.00042-9.
6. Kidney failure. Gov.au. Accessed November 15, 2023. https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/kidney-failure.
7. Abraham K, Penczynski K, Monien BH, et al. Risks of misinterpretation of biomarker measurements in spot urine adjusted for creatinine - A problem especially for studies comparing plant based with omnivorous diets. Int J Hyg Environ Health. 2023;249:114142. doi:10.1016/j.ijheh.2023.114142.
8. Types of blood pressure medications. www.heart.org. Accessed November 15, 2023. https://www.heart.org/en/health-topics/high-blood-pressure/changes-you-can-make-to-manage-high-blood-pressure/types-of-blood-pressure-medications.
9. Nachawati D, Patel JB. Alpha-Blockers. StatPearls Publishing; 2023.
10. Hui C, Khan M, Khan Suheb MZ, Radbel JM. Diabetes Insipidus. StatPearls Publishing; 2023.
11. Li Y, Yu T, Liu Z, et al. Association of Serum Uric Acid, Urea Nitrogen, and Urine Specific Gravity Levels at 16-18 Weeks of Gestation with the Risk of Gestational Diabetes Mellitus. Diabetes Metab Syndr Obes. 2020;1;13:4689-4697. doi:10.2147/DMSO.S282403.
12. Imran S, Eva G, Christopher S, Flynn E, Henner D. Is specific gravity a good estimate of urine osmolality? J Clin Lab Anal. 2010;24(6):426-30. doi:10.1002/jcla.20424.
13. The Editors of Encyclopedia Britannica. refractive index. In: Encyclopedia Britannica. ; 2023.
14. Yang Q, Zhang AH, Miao JH, et al. Metabolomics biotechnology, applications, and future trends: a systematic review. RSC Adv. 2019;14;9(64):37245-37257. doi:10.1039/c9ra06697g.
15. Stevens VL, Hoover E, Wang Y, Zanetti KA. Pre-Analytical Factors that Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites. 2019;25;9(8):156. doi:10.3390/metabo9080156.
About the Author

Hillary Threatt CPhT, MDT (AMT), MSc
is a medical technologist working at Quest Diagnostics laboratories. Much of Hillary’s time is spent working in clinical chemistry, hematology, coagulation, urinalysis, and blood banking. Her national certificates include molecular diagnostics technologist and pharmacy technician. With eight years of medical experience under her belt, Hillary’s experiences also extend to research working under IRB protocol, and a Biotechnology master’s thesis based on identifying mechanisms in Enterobacterales.