Monoclonal antibody therapy: What compatibility testing labs need to know
Earning CEUs:
For a printable version of the October CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
OCTOBER LEARNING OBJECTIVES
1. Discuss the discovery of immune therapy and the diseases that specific anti-CD markers can treat.
2. Recall the immune mechanisms involved in CD38 and CD47 therapy.
3. Describe the blood banking testing problems associated with CD38mAb therapy and techniques used to mitigate them.
4. Describe the blood banking testing problems associated with CD47mAb therapy and techniques used to mitigate them.
In 2015, Darzalex (Daratumumab or “Dara”) became the first human CD38 mAb approved by the FDA for the treatment of relapsed or refractory multiple myeloma in patients who have received at least three prior treatments.5 Other anti-CD38 agents, such as MOR202, are in trials. In June 2019, the FDA approved a combination of Darzalex-Revlimid-dexamethasone to treat newly diagnosed multiple myeloma (MM) patients with who failed to qualify for autologous stem cell transplant.2 Isatuximab (SAR-650984) is another CD38 IgG1 mAb in clinical trials for the treatment of patients with MM. It received orphan designation for relapsed/refractory multiple myeloma by the FDA in the second quarter of 2019.3
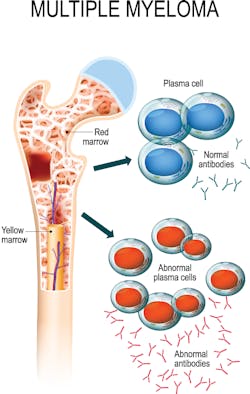
CD47 mAbs are currently undergoing clinical trials for multiple solid and hematologic malignancies, (e.g., Hu5F9-G4, CC-90002, SFR231) and in 2018 the FDA granted SFR231 orphan drug designation for MM.4 Refer to Figure 1 of a bone marrow aspirate with plasma cells in MM.
CD38 and CD47 overview
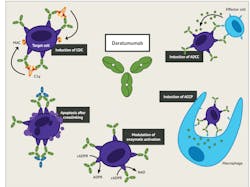
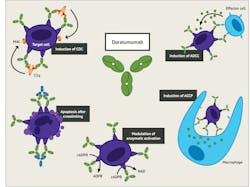
CD38 is a type II transmembrane glycoprotein and found in low levels on the surface of many types of cells including B and T lymphocytes, natural killer (NK) cells, plasma cells, platelets, and red blood cells (RBCs). It functions as a receptor and adhesion molecule, as well as a manufacturer and hydrolyzer of an intracellular calcium ion-activating messenger. CD38 is highly expressed on plasma cells in MM; this makes it a desirable target for MM therapy. The binding of CD38 mAb to the CD38 receptor inhibits the growth and proliferation of MM cells and induces tumor cell death or apoptosis through a variety of immunological mechanisms. This includes Fc-dependent immune-effector mechanisms, direct apoptotic activity, and immunomodulatory effects by the elimination of CD38-expressing regulatory T, regulatory B, and myeloid-derived suppressor cells.6,7 (Figure 2)
Expressed on virtually all tissue and cell types including RBCs and platelets, CD47 has many functions including cell migration, cell adhesion, and T cell function. CD47 is an integrin-associated transmembrane protein (IAP) that has high affinity for thrombospondin (TSP) and signal-regulatory protein alpha (SIRPα) on the macrophage membrane that is directly involved in the prevention of phagocytosis by macrophages.8 This “don’t eat me” signal protects transfused RBCs and platelets from rapid elimination by macrophages in the spleen. CD47 is also a transmembrane protein and part of the Rh complex. The expression of CD47 is affected by Rh phenotype; rr (dce/dce) having the highest expressions, compared to D-positive, and Rhnull having the least. Studies demonstrate that CD47 expression decreases as RBCs age, so it additionally functions as a “eat me” signal and considered a marker of senescence.9,10
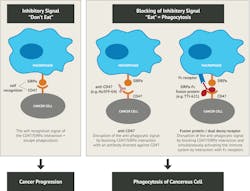
A cell surface protein called calreticulin (CRT) appears to be expressed on malignant cells, but not on most normal cells. As cancer cells are dying, (e.g., during chemotherapy treatment) CRT is upregulated and emits “danger” signals (part of the warning system known as damage-associated molecular patterns or DAMPs). CRT interacts with pattern recognition receptors (PRRs) expressed on myeloid and lymphoid cells, such as low-density lipoprotein receptor-related protein 1 (LRP-1), to promote phagocytosis. Also highly expressed on cancer cells and in opposition, the CD47-SIRPα pathway impedes macrophage-mediated destruction. As a therapeutic human monoclonal IgG4 antibody, anti‐CD47 (e.g., Hu5F9‐G4) blocks interaction of CD47-SIRPα, allowing the pro-phagocytic calreticulin/LRP-1 collaboration to facilitate the “eat me” signal. As a consequence, treating patients with anti-CD47 paves the way for clearing malignant cells. Refer to Figure 3 for proposed mechanism of action of CD47‐ targeted anti‐tumorAlthough new monoclonal antibodies show promise for improved outcomes, patients receiving these treatments for MM and other hematological diseases present many challenges for both technologists performing pretransfusion compatibility testing and physicians that interpret the clinical significance of the results.
Interference in blood bank testing when samples contain anti-CD38
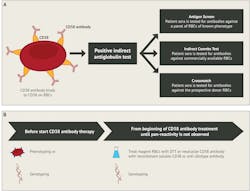
After treatment with anti-CD38 commences, immediate spin reactions are unaffected, but residual immunotherapeutic antibody in the patient’s plasma cross-reacts with RBCs. This causes unexpected, generally weak, panreactive serologic reactions when utilizing the indirect antiglobulin test (IAT) (e.g., antibody screens, antibody identification panels, phenotype tests, and crossmatches). Interference is seen with all media (e.g., saline, LISS [low-ionic-strength solution], and PeG [polyethelene glycol]), by all common test methods (tube, gel, and solid phase), and can persist for six months following treatment.14,15 The autocontrol (AC) and direct antiglobulin test (DAT) may be positive or negative (most often), and results frequently resemble an antibody to a high incidence antigen or a warm autoantibody. Adsorptions using untreated or chemically treated cells are not effective.
Mitigation of anti-CD38 interference
The goal of any blood bank technologist when faced with panreactivity due to mAb treatment is to ensure clinically significant alloantibodies are not underlying the drug antibody. The most practical and inexpensive method employed to eliminate the interference of anti-CD38 is pretreating reagent red cells with dithiothreitol (DTT). DTT is a reducing agent that disrupts disulfide bonds formed between cysteine residues. The CD38 molecule (also on RBCs) has five disulfide bonds, so when a patient’s plasma is tested with reagent red cells treated with DTT, reactivity from anti-CD38 is abolished. It is important to keep in mind that Kell, Knops, Dombrock, Lutheran, Yt (formerly Cartwright), and JMH blood group system antigens, as well as the LWa antigen, are destroyed by DTT, which cannot be excluded during antibody identification.
Generally used by large reference laboratories, the patient’s plasma may be tested with a panel of cord RBCs that have been extensively phenotyped.16 Cord RBCs have very little CD38, if expressed at all. Recombinant soluble human CD38 or anti-daratumumab idiotype can be utilized to eliminate interference, but is not widely available at this time.14
Interference in blood bank testing when samples contain anti-CD47
Once treatment with anti-CD47 begins, false positive reactions can be seen in all phases of testing (immediate spin, 37°C, and IAT), with all media (e.g., saline, LISS, and PeG), and with all methods of IAT testing (i.e., tube, gel, solid phase). ABO discrepancies occur due to false positive results in the reverse typing (all types except for O), and false positive results in the forward typing due to spontaneous agglutination of patient RBCs. Because of the high level of CD47 on RBCs, these reactions may mimic those seen with IgM antibodies. Cord RBCs are also reactive in all phases. DATs may be falsely negative due to a “blocking effect” caused by high levels of antibody present, but eluates are strongly positive. False negative phenotyping test results can occur due to RBCs heavily coated by anti-CD47.
Mitigation of anti-CD47 interference
The most practical and cost- effective method currently available to eliminate the interference of anti-CD47 is to utilize an anti-IgG reagent that does not bind to IgG4, such as anti-IgG (Murine Monoclonal) Gamma-clone (Hu5F9-G4 and CC-90002 are monoclonal IgG4 anti-CD47). Occasionally, even using this method, it may still demonstrate a weak reactivity because of carryover to the IAT phase of testing. Allogenic adsorption or adsorption using pooled platelets or papain treated RBCs generally require four adsorptions.11
What compatibility testing labs and clinicians need to know
Without a medication history when samples are submitted for work-up, regardless of the presentation, a blood bank technologist may be confounded and unknowingly waste precious time and resources chasing “ghosts.” With this said, it is very important that clinicians and other healthcare providers understand that pretransfusion testing, including an extended phenotype should be performed before initiating treatment (either serology or predictive genotype for D, C, c, E, e, K, Jka, Jkb, Fya, Fyb, S, and s antigens). In addition, a complete, accurate history (e.g., diagnosis, medications, transfusions, transplants, and pregnancies) must be provided any time pretransfusion testing and/or antibody identification is required. This information can be instrumental in helping the blood bank technologist provide the safest donor red blood cells for transfusion. As with any other emergent acute, life-threatening circumstance, group O-negative or O-positive RBCs can be transfused with physician approval, following institutional protocols.
Similar to interfering in RBC antibody detection, plasma containing anti-CD38 or anti-CD47 may also affect detection of antibodies to HLA Class I and platelet antigens, as well as interfere with platelet crossmatch testing. CD47 is weakly expressed or absent from the rare Rhnull cells.8,17 Clinically significant alloantibodies may be masked by the presence of monoclonal antibodies. More extensive testing beyond standard methods can lead to delays in providing RBCs for transfusion. Hospitals must communicate to the compatibility testing laboratory when patients are expected to begin monoclonal antibody therapies.
In conclusion
Rapid development and use of monoclonal antibodies continue to be investigated for clinical applications, such as acute lymphoblastic leukemia (ALL), lymphomas, and acute myeloid leukemia (AML), as well as in solid tumors. Unfortunately, interference of such immunotherapies on pretransfusion testing results is often missed or insufficiently investigated. The impact on blood bank testing is only recognized when patients require a blood transfusion, yet clinically significant alloantibodies fail to be eliminated using routine test methods. Blood bank technologists and other healthcare providers must stay vigilant to remain up-to-date on published literature, workshops, and other educational venues for best practices to mitigate mAb interference during such testing.
REFERENCES
- Engel P, Boumsell, L, Balderas R, et al. CD Nomenclature 2015: Human Leukocyte Differentiation Antigen Workshops as a Driving Force in Immunology. J Immunol. 2015; Nov 15;195(10):4555-63.
- U.S. Food and Drug Administration. FDA approves daratumumab for multiple myeloma ineligible for autologous stem cell transplant. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-daratumumab-multiple-myeloma-ineligible-autologous-stem-cell-transplant. Published June 28, 2019. Accessed August 26, 2019.
- Sanofi. FDA to review isatuximab as a potential treatment for relapsed/refractory multiple myeloma. http://www.news.sanofi.us/2019-07-10-FDA-to-review-isatuximab-as-a-potential-treatment-for-relapsed-refractory-multiple-myeloma. Published July 10, 2019. Accessed August 26, 2019.
- Holland PM, Normant E, Adam A, et al. CD47 Monoclonal Antibody SRF231 Is a Potent Inducer of Macrophage-Mediated Tumor Cell Phagocytosis and Reduces Tumor Burden in Murine Models of Hematologic Malignancies. Blood. Dec 2016; 128(22):1843. https://www.surfaceoncology.com/wp-content/uploads/Surface-ASH-Poster-11.29-FINAL.pdf. Accessed August 26, 2019.
- Novel Drug Approvals for 2015. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2015. Accessed August 27, 2019.
- van de Donk NWCJ, Janmaat ML, Mutis T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016; 270(1):95–112. https://onlinelibrary.wiley.com/doi/pdf/10.1111/imr.12389.
- van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018; 131(1):13-29. http://www.bloodjournal.org/content/bloodjournal/131/1/13.full.pdf?sso-checked=true. Accessed August 26, 2019.
- Pretini V, Koenen M, Kaestner L, et al. Red Blood Cells: Chasing Interactions. Frontiers in Physiology July 2019 Vol.10 article 945. https://www.frontiersin.org/articles/10.3389/fphys.2019.00945/full. Accessed August 28, 2019.
- Sosale N, Discher DE. Marker-of-self becomes marker-of-senescence. Blood. 2012;119(23):5343-5344. http://www.bloodjournal.org/content/bloodjournal/119/23/5343.full.pdf. Accessed August 26, 2019.
- Burger P, Hilarius-Stokman P, de Korte D, et al. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood. Jun 2012;119(23):5512-5521. http://www.bloodjournal.org/content/bloodjournal/119/23/5512.full.pdf. Accessed August 26, 2019.
- Velliquette RW, Aeschlimann J, Kirkegaard J, et al. Monoclonal anti‐CD47 interference in red cell and platelet testing. Transfusion. 2019; 59(2):730-737. https://onlinelibrary.wiley.com/doi/pdf/10.1111/trf.15033. Accessed August 26, 2019.
- Liu X, Kwon H, Li Z, et al. Is CD47 an innate immune checkpoint for tumor evasion? J Hematol Oncol. 2017;10(1):12. Published 2017 Jan 11. https://jhoonline.biomedcentral.com/articles/10.1186/s13045-016-0381-z?optIn=true. Accessed August 26, 2019.
- Chao MP, Jaiswal S, Weissman-Tsukamoto R, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2(63):63ra94. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4126904/. Accessed August 19, 2019.
- Oostendorp M, Lammerts van Bueren JJ, Doshi P, et.al. When blood transfusion medicine becomes complicated due to interference by monoclonal antibody therapy. Transfusion. 2015 Jun; 55:1555-62.
- Regan DM, Markowitz MA. AABB Association Bulletin #16-02. Mitigating the Anti-CD38 Interference with Serologic Testing. http://www.aabb.org/programs/publications/bulletins/Documents/ab16-02.pdf. Published January 15, 2016. Accessed August 26, 2019.
- Schmidt AE, Kirkley S, Patel N, et al. An alternative method to dithiothreitol treatment for antibody screening in patients receiving daratumumab (Letter). Transfusion 2015;55(9):2292-2293.
- Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000;95:375-387. http://www.bloodjournal.org/content/95/2/375. Accessed August 26, 2019.
About the Author

Wyenona Hicks, MS, MT(ASCP) SBB
Wyenona Hicks, MS, MT(ASCP) SBB, serves as Program Manager of Medical Technology Education, OneBlood, Inc.

Juan A. Merayo-Rodríguez, MD
serves as Medical Director, LifeSouth Community Blood Centers, Inc.