Artificial intelligence and digitalization are revolutionizing laboratory diagnostics
So much is in silos across diagnostic laboratories: data, analyzers, devices—even people—divided by walls, by processes, by distance or simply by force of habit. The result can be partial visibility, limited insight and duplication of effort. Artificial intelligence (AI) and digitalization are proving to be the tools to help transcend these barriers. According to a 2018 survey of 200 laboratory executives, 69% expect widespread adoption of AI in the IVD lab within four years.1
While the workload is growing, diagnostics and treatment are also becoming more complex, relying on and producing massive amounts of data. Healthcare providers are now looking to AI to operationalize the data to support more-objective, data-driven treatment decisions that are tailored to the needs of each patient.
Digitalization is the foundation
Automation and diagnostic software have been commonplace in the lab for years. But many tasks are still performed manually with no digital records. Even if the data is electronic, often it is in a format that cannot easily be exchanged with other systems. In fact, 80% of data remains unstructured2 and stored away in closed systems that are physically or technically disconnected from each other. To effectively use this information, it must be made interoperable, e.g., in a secure, open-sourced, vendor-neutral environment that can synthesize from disparate sources and systems. Digitalization is transforming these isolated and often-hidden sources of information into structured, sharable, actionable data.
Labs today use software to automate management of samples, operations and results to optimize workflow and staff utilization. For example, rules-based autoverification evaluates patient results against multiple parameters to validate and speed up reporting or reflex actions. Concurrently, intelligent systems actively analyze operations to predict bottlenecks and warn of potential issues, such as STAT sample delays or impending reagent expiration. Beyond the core lab, healthcare systems rely on digitalization to manage hundreds or thousands of point-of-care (POC) testing devices and their data. These rules-based applications follow predefined logic, performing tasks and calculations explicitly as they are programmed. AI is the next step in the evolution of laboratory software.
Today and tomorrow: what AI brings to the lab
Artificial intelligence-based software uses neural networks designed to emulate human thought processes. AI can recognize patterns beyond defined rules and analyze significantly higher volumes of information than humans could manage. Consequently, the diagnostics IT of tomorrow will build on and supercharge the capabilities of the technology we work with today. For example:
Streamlining laboratory operations
System failures are disruptive and costly. Today, AI can reduce unscheduled downtime by monitoring critical analyzer components in real time to detect system failures weeks before they occur. Imagine a future in which AI monitors predictive maintenance data, as well as inventory consumption rates, supply chain information and disease trends to automatically schedule just-in-time delivery of replacement parts, reagents and consumables. AI could also schedule repairs or installations to be completed in the most convenient and effective manner, whether by remote assistance or in-person service.
Process-management systems today can visualize lab operations from a centralized dashboard to provide real-time status and analyze trends across all tests, connected analyzers and locations within a health system. These systems can uncover inefficiencies and optimize clinical operations. With AI, visibility could be expanded across geographies, health systems and modalities to establish and benchmark best practices and expertise, and thereby, improve productivity and staff utilization.
To reduce manual tube handling and sorting, today’s AI-enabled camera systems can instantly identify and characterize each tube using machine learning trained on an extensive image library of container types. Cameras detect sample container parameters, and the sample-handling system dynamically adjusts to meet the unique needs of each tube, such as routing and STAT prioritization, aspiration position or special handling requirements for tube-top sample cups or pediatric samples. Innovations in computer vision will transform many operational tasks that previously relied on human sight, but also hold incredible potential for performing clinical analysis.
Clinical analysis and decision support
Digital microscopy analysis leverages machine-learning algorithms trained using tens of thousands of specimen images. It can quickly, consistently and accurately identify and classify particulate and cellular objects in urine, serum or tissue samples, emulating and augmenting the expertise of humans, while easily scaling to support higher volume. With AI, images could be compared with specimens from across health systems, demographics and geographies to identify and diagnose more rare diseases.
Today, the majority of patient results are quickly and consistently autoverified and reported to physicians with minimal human intervention. In the future, AI has the potential to expand precision medicine by adapting reference ranges and reflex-testing guidelines to the unique needs of each patient. To accomplish this, patient and LIS data would be augmented with insights from demographic, population and diagnostic outcomes data.
Further expanding personalization, clinical decision-support systems are currently using AI to aggregate individual patient results from imaging, laboratory, pathology and genetic testing. In coming years, AI could integrate these sources with clinical studies and population health data to recommend treatment pathways and provide more-comprehensive insight to help physicians make best-practice decisions.
Can artificial intelligence influence changes in testing and treatment protocols?
Sepsis afflicts more than 1.5 million patients annually in the U.S., killing over 250,000, and is responsible for one out of every three hospital deaths.3 This is well-known. But what if AI could reveal just how pervasive fatal sepsis outcomes are across disease states? How might this influence testing and treatment protocols?
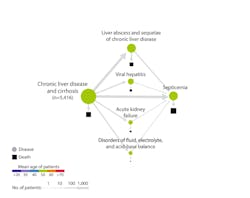
Atul Butte, MD, PhD, chief data scientist at University of California Health, recently presented findings4 that applied artificial intelligence to analyze data from 10.4 million patients from 350 California hospitals. Disease-progression mapping for 300 disease states revealed some surprising trajectories. Chronic liver disease and myocardial infarction are common morbidities, but unexpectedly, both led to death from sepsis; in other words, the ultimate cause of death for these patients with liver and heart conditions was an infectious disease, not a primary problem in the liver or heart. (Figure 1)
With mortality from sepsis increasing 8 percent for every hour treatment is delayed, as many as 80 percent of sepsis deaths could be prevented with rapid diagnosis and treatment. Findings like these have the potential to drive testing protocols. As an illustration, hospital admission guidelines might indicate procalcitonin (PCT) testing for the early identification of sepsis.
Advanced data-mining analytics such as those presented by Dr. Butte are increasingly being used to identify often-unexpected disease associations, and fill gaps in our current medical understanding. These insights can help physicians determine the appropriate protocols – such as diagnostic tests like high-sensitivity cardiac troponin or noninvasive blood tests based upon serum markers of liver fibrosis assays – to aid in identification of at-risk patients. Then, through intervention, physicians can alter the disease trajectory, potentially reducing the incidence of heart attacks, advanced liver disease or sepsis. While this technology remains nascent, such data-driven insight holds tremendous potential to inform diagnostic and clinical models. (Figure 2)
Intelligence-based medicine
Enhancing our digital capabilities with artificial intelligence can be daunting, but this evolution holds incredible potential to harness the power of information to help guide clinical decisions.
Anthony Chang, chief intelligence and innovation officer at Children’s Hospital of Orange County, summed it up well: “For us to fulfill our vision of precision medicine and population health, we need to change the paradigm of evidence-based medicine to that of a data-science-driven, intelligence-based medicine.”
REFERENCES
- Future of Artificial Intelligence in the Diagnostic Lab survey. Commissioned by Siemens Healthineers. 2018. Available from: https://www.siemens-healthineers.com/en-us/news/mso-ai-will-change-clinical-laboratory.html
- https://www.ibm.com/blogs/watson/2016/05/biggest-data-challenges-might-not-even-know
- Hajj J, Blaine N, Salavaci J, Jacoby D. The “centrality of sepsis”: a review on incidence, mortality, and cost of care. Healthcare (Basel). 2018 Sep;6(3):90. doi:10.3390/healthcare6030090. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164723
- Paik H, Kan MJ, Rappoport N, et al. Tracing diagnosis trajectories over millions of patients reveal[s] an unexpected risk in schizophrenia. Sci Data. 2019;6(201). doi:10.1038/s41597-019-0220-5. Available from: https://www.nature.com/articles/s41597-019-0220-5
About the Author

Stuart Kerty
serves as the Global Solutions Marketing Manager for Siemens Healthineers.