Clairity becomes the first FDA authorized AI platform for breast cancer prediction
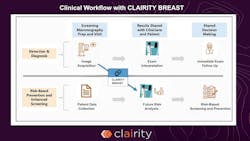
Clairity, Inc. has received U.S. Food and Drug Administration (FDA) De Novo authorization for CLAIRITY BREAST, a novel, image-based prognostic platform designed to predict five-year breast cancer risk from a routine screening mammogram.
With this authorization, Clairity is planning to launch among leading health systems through 2025.
CLAIRITY BREAST analyzes subtle imaging features on screening mammograms that correlate with future breast cancer risk, making early risk prediction feasible based on a screening mammogram alone. The result is a validated five-year risk score delivered to healthcare providers through existing clinical infrastructures, supporting more personalized follow up care.
Be the first to know when it launches, and how you can get it