Papillomaviruses are a large family of small viruses specific to their hosts1 that are detectable in a plethora of animal species, including humans.2 In the case of humans, more than 200 types of human papillomavirus (HPV) have been identified and among those, 85 genotypes are characterized.1 The link between HPV and cervical cancer was discovered in the 1980s by Harald zur Hausen—a discovery that led to the 2008 Nobel Prize for Physiology or Medicine.3 Over the next decade, more evidence would continue to confirm the link between certain HPV genotypes and cervical cancer. Thus far, 14 genotypes of HPV, known as the high-risk HPVs, are linked to cancer, with HPV 16 and 18 causing 70 percent of cervical cancers and pre-cancerous cervical lesions.4 Fortunately, infection does not always lead to cancer. Some women are infected with these high-risk strains but remain asymptomatic. Their immune system may even clear itself of infection altogether. However, for other women, the persistent infection does cause medical problems.
Worldwide, cervical cancer is the fourth most prevalent cancer in women. In 2018, it was estimated that 570,000 new cases of this disease were diagnosed, and cervical cancer accounts for approximately 7.5 percent of all female cancer deaths.4 HPV is most often contracted through sexual contact. However, skin-to-skin contact is a well-recognized mode of transmission as well. Not surprisingly then, there is evidence that HPV can also play a role is some other cancers including rectal, vulval, vaginal, penile and oropharyngeal cancers.4 However, to date, cervical cancer is the most studied cancer linked to this viral infection. This is in part due to the availability of extensive screening methods and the evolution of our understanding of how certain strains of HPV can induce carcinogenesis.
The emergence of cytological evaluation
There is a significant latency period between infection and the development of cervical cancer or pre-cancerous lesions. This led doctors to ask, “Would it be possible to detect infection early and pre- emptively treat the patient to minimize the risk of danger?” Dr. Georgios Papanicolaou found that abnormalities in cellular morphology could be observed when examining cells collected from a women’s cervix under the microscope. The first mass screening program was launched in 1952 in Tennessee. Since then, it has been estimated that the Pap smear test has successfully prevented 105,000 to 492,000 cases of cervical cancer in the United States over the past three decades.5 While cytological examination can allow a pathologist to infer the presence of HPV based on morphological abnormalities, diagnosis relies heavily on molecular biology techniques, allowing identification of the HPV strain(s) and subsequent classification of the infection as high risk or low risk.
Nucleic acid hybridization methods
The emergence of molecular techniques enabled the detection of HPV strains that have a high association with cervical cancer. Original nucleic acid hybridization methods, such as Southern blotting, in situ hybridization (ISH), and dot blot hybridization, used radioactive nucleic acid probes to detect the presence of HPV.6 Each of these hybridization methods takes advantage of the complementarity of nucleic acid bases. By applying high temperatures and detergents, it is possible to denature the DNA inside of a cell, introduce the radiolabeled DNA probes, and with slow cooling, renaturation of the probe to the native DNA strands.7 However, these protocols were very time-consuming and radioactivity is harmful to human health. Thus, a need for faster, non-radioactive methods arose.
First non-radioactive testing method
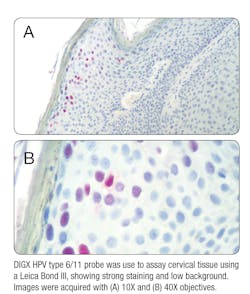
The first non-radioactive ISH probe, made available in the 1980s, answered this need. It provided a safe, faster, and highly sensitive and specific method for detecting the presence of HPV DNA in situ.8 One of the biggest advantages of ISH is that it is easily applied to tissues that have been fixed and processed. It also allows you to simultaneously assess the localization of HPV DNA in the cellular nuclei of your sample while observing the cells for any morphological abnormalities. ISH can be performed using either DNA or RNA oligonucleotide probes. While DNA probes have been the “gold standard” for the last four decades, the use of RNA probes provides several key advantages over DNA. First, RNA probes can be synthesized easily in vitro by transcription, enabling efficient synthesis of large amounts of continuously labeled probes of uniform lengths.9 These RNA probes form more stable hybrids with the target RNA or DNA, which results in improved sensitivity.
Polymerase chain reaction
In situ PCR is a powerful method whereby low copy amounts of DNA or mRNA can be amplified and measured using polymerase chain reaction (PCR) or reverse transcriptase PCR (RT-PCR), respectively, in frozen or paraffin-embedded tissue sections or cell suspensions. Amplicon detection is then accomplished using labeled probes. There are several PCR-based methods that can be used to identify the genotype of HPV, and thus infer the treatment needs for that patient. These techniques are highly sensitive, specific and commonly used. Conventional PCR assays use consensus primers that enable amplification of many HPV genotypes at once. These primers target the homologously conserved regions of the HPV genome that are similar across most strains. After amplification, the individual genotypes can be ascertained using several different techniques, such as restriction fragment length polymorphism (RFLP) analysis, linear probe assays, direct sequencing, or genotype-specific primers. However, PCR is not fail-safe and false negative results can occur, particularly with samples that are infected with multiple HPV genotypes and have a low viral copy number.6
In vitro RNA-RNA hybridization
As technology continues to evolve, other methods continue to emerge and improve our ability to reliably diagnose and treat individuals infected with high-risk strains of HPV. Currently, there is an in vitro DNA-RNA hybridization methodology that is one of the two FDA-approved tests for diagnostic testing in the U.S. This system uses a non-radioactive signal amplification method based on the hybridization of the target HPV-DNA to labeled RNA probes in solution. Specific antibodies attached to the well of a microtiter plate then capture the DNA-RNA hybrids formed in solution. Luminescence is then used to detect the captured hybrids. The strength of the emission can be directly correlated with the concentration of the target DNA present in the specimen. While this assay cannot genotype individual HPV strains, it does differentiate between high-risk and low-risk infections. Furthermore, this assay can be fully automated, reducing the risk of human error in sample processing.6
Detection of mRNA expression
HPV RNA has become an area of interest as a target for molecular diagnosis of HPV infection. Rather than testing for the presence of viral genomes, assessing the level of viral mRNA expression measures the viral activity in the infected cells. This can be very useful when trying to assess the severity of the infection, particularly if the HPV strain is one of the 14 cancer-causing strains. PCR-based methods, including RT-PCR, could be used to measure the levels of mRNA expression.10 Another emerging method uses flow cytometry to detect the presence of HPV mRNA in whole cells. It combines the in situ hybridization technology with flow cytometry analysis, whereby the probe does not emit a fluorescent signal unless it is bound to its target sequence. Using this technique, the overexpression of HPV oncogenes can be assessed, and a prediction can be made on the likelihood of cervical cancer progression.11
Beyond cervical cancer
As mentioned earlier, HPV can be linked to several other cancers, including cancers at extragenital sites such as anal/rectal cancers, oropharyngeal cancer and penile cancers. In recent studies, it has been reported that the incidence of sexually transmitted infections (STIs) at extragenital sites, including the oropharynx and rectum, is on the rise.12 While the discussion has been predominantly centered on extragenital gonorrhea and chlamydia infections, it raises awareness for the need of routine screening at extragenital sites. Like HPV, gonorrhea and chlamydia can often be asymptomatic.
As it stands today, the only approved HPV screening methods center around cervical screening. There are no CDC guidelines for HPV screening at extragenital sites in men and women; or urogenital sites in men.13 Also there is no approved test currently for HPV detention in the mouth or throat.13 Establishing testing methods to screen for HPV infections at these extragenital sites will be paramount in our continued battle against HPV infection-induced cancers. In conclusion
Technology clearly evolves over time, improving our ability to diagnose and treat infections appropriately. Physicians can use the current available testing methods to not only diagnose HPV infection early on, but to predict the likelihood that the infection could one day turn into cancer. However, much work is still needed to improve HPV screening beyond the cervix, so that we can catch infections of the penis or extragenital sites early on, and use the same preemptive strike approach for other HPV-related cancers that physicians have been using to combat cervical cancer for the last six decades.
REFERENCES
- Burd E.M. (2003, January). Human Papillomavirus and Cervical Cancer. Clinical Microbiology Review, 1-17. doi:10.1128/CMR.16.1.1–17.2003.
- Fe´lez-Sa´nchez M., Bravo, I.G. (2015). Papillomaviruses: Viral evolution, cancer and evolutionary medicine. Evolution, Medicine, and Public Health, 32-51. doi:10.1093/emph/eov003.
- The Editors of Encyclopaedia Britannica. (2019, March 7). Harald zur Hausen. Retrieved from Encyclopædia Britannica: https://www.britannica.com/biography/Harald-zur-Hausen
- World Health Organization. (2019, January 24). Human papillomavirus (HPV) and cervical cancer. Retrieved from World Health Organization: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
- Wong S. (2019, May 13). Georgios Papanikolaou: inventor of the pap smear cervical cancer test. Retrieved from NewScientist: https://www.newscientist.com/article/2202635-georgios-papanikolaou-inventor-of-the-pap-smear-cervical-cancer-test/
- Abreu A. L., Souza R.P., Gimenes F., Consolaro M.E. (2012). A review of methods for detect human papillomavirus infection. Virology Journal, 262. doi:10.1186/1743-422X-9-262.
- Kapranos N.C. (1991). New methods of HPV identification. Journal of the European Academy of Dermatology & Venereology, 145-152.
- Todd J.A., Jou L., Shen J.T., et al. (1989). A rapid DNA probe test for detecting human papilloma virus types 6/11 and 16 in biopsy specimens. Molecular Cell Probes, 273-288.
- Farrell R.E. (2010). RNA Methodologies. Elsevier Inc.
- Villa L.L., Denny L. (2006). Methods for detection of HPV infection and its. International Journal of Gynecology and Obstetrics, S71-S80.
- Kottaridi C., Tsiodras S., Spathis A., et al. (2011) Clinical performance of human papilliomavirus E6, E7 mRNA flow cytometric assay compared to human papillomavirus DNA typing. Analytical and Quantitative Cytology and Histology, 305-310.
- Schmidt V. (2019). STI Incidence on the Rise. Clinical Lab Products - Disease States: http://www.clpmag.com/2019/10/sti-incidence-rise/
- Centers for Disease Control and Prevention. (n.d.). Genital HPV Infection Fact Sheet. Retrieved from Centers for Disease Control and Prevention: https://www.cdc.gov/std/hpv/stdfact-hpv.htm
About the Author

Kayla M. Hager
serves as Technical Marketing Manager at Enzo Biochem, Inc, with over five years of industry experience. Previously, she was in graduate school investigating the non- canonical functions of the p53 tumor suppressor gene. She holds a B.S. in Genetic Engineering, a MA and a MPhil in Genetics and Development.