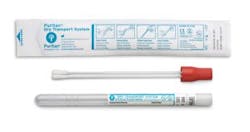
Puritan’s U.S. made patented HydraFlock swabs are specimen collection devices for genetic testing. These swabs are manufactured using a proprietary process to deliver optimum collection and release properties. Polyester flock fibers are multi-length and designed to produce tips with maximum surface area. Puritan’s 25-3306-2H BT is a dual swab, two 6” standard tip swabs in a dry transport tube, held securely in a snug fitting cap for reliable transport to the lab. Other swab configurations include molded break points and smaller tips for naso-pharyngeal as well as pediatric applications. Puritan Medical Products
SoftGenetics’ NextGENe software provides a user-friendly solution for next generation sequencing analysis compatible with all NGS instruments including Illumina and Ion Torrent systems.
NextGENe software has been validated by hundreds of clinical users. The Windows-based software includes tools for SNP/Indel Detection, Copy Number Variation analysis, RNA-Seq, HLA typing, and more. Results are displayed in the interactive NextGENe Viewer offering detailed visualization and flexible reporting options. The built-in AutoRun tool facilitates performing data analysis in large batches, with streamlined project setup and automatic unattended processing, which, in combination with Geneticist Assistant Interpretive Workbench, forms a complete NGS pipeline. SoftGenetics
Genetic research and testing produce large amounts of data that must be analyzed, stored, and correlated; not only with drug reactions, but also with numerous patient characteristics. Attempting to accomplish this using manual methods or basic electronic applications is neither cost-effective nor efficient. SCC’s Genetics Information Systems Suite supports the analysis, storage, and correlation efforts, and establishes the large number of possible test protocols. These software solutions are capable of providing online availability of the most current test interpretations and recommendations, which is crucial for the successful practice of personalized medicine. Soft Computer
AutoGenomics Inc. has developed INFINITI NeuR Assay that detects mutations in 16 genes in the brain reward pathway:
• Serotonin 2A Receptor • Dopamine D1 Receptor • Methylene Tetrahydrofolate Reductase • Mu Opioid Receptor • Serotonin Transporter • Dopamine D4 Receptor • Kappa Opioid Receptor • Galanin • Catechol-O-Methyltransferase • Dopamine Transporter • Gamma-Aminobutyric Acid (GABA), • Delta Opioid Receptor • Dopamine D2 Receptor • Dopamine Beta Hydroxylase • Mu Opioid Receptor • ATP Binding Cassette Transporter 1 (ABCB1)
The test can be performed on AutoGenomics’s INFINITI PLUS and INFINITI High Throughput Systems (HTS). INFINITI NeuR Assay is for Research Use Only and not for use in diagnostic procedures. AutoGenomics is planning to file for 510K for this assay. Publications show that mutations in these genes can help to determine a person’s risk of opioid addiction with pain medication, and provide guidance for a better treatment plan. In AACC 2017, AutoGenomics received the Industry Division Poster Award for their Opioid Addiction Panel Study. AutoGenomics
DiaSorin Molecular announced the U.S. introduction of the Simplexa C. difficile Direct Assay, upon receiving clearance from the Food and Drug Administration. The new assay runs on the company’s LIAISON MDX qPCR system, a scalable benchtop instrument that delivers qualitative and quantitative, sample-to-answer, multi-analyte results.
The Simplexa assay detects the Clostridium difficile toxin B gene (tcdB), present in liquid or unformed stool samples, aiding in the diagnosis of C. difficile infection.
According to the U.S. Centers for Disease Control and Prevention, approximately 15,000 U.S. deaths are attributed to C. difficile infection each year, and studies indicate that C. difficile is now the most common microbial cause of infections in U.S. hospitals. It is estimated that this infection costs $4.8 billion each year in the U.S. alone. Diasorin Molecular
New Products
Primera’s LX500 color label printer may increase the efficiency of your lab while reducing the risk of misidentification of specimens. Full-color printing adds high impact to labels and helps reduce errors by eliminating handwriting. Print text, graphics, color alerts, and logos on water-resistant material, along with high-resolution linear and 2D barcodes in seconds.
Primera
The Lab Draw Answer Book answers over 400 commonly asked questions on drawing blood samples for laboratory testing. This essential desk reference is intended for all healthcare professionals who draw blood specimens for laboratory testing including laboratory personnel, nursing professionals, medical assistants, and their educators.
Originally published as Blood Specimen Collection FAQs, this second edition is renamed and completely updated with over 100 new entries. Each answer is highly researched and reflects the latest standards from the Clinical and Laboratory Standards Institute, the Infusion Nurses Society, and OSHA. Published by the Center for Phlebotomy Education, this 440-page reference covers all aspects of preanalytical processes, education, and management.
Center for Phlebotomy Education
HYPE-WIPE bleach towelettes are EPA registered in all states as a one-step disinfectant with a one- minute kill time for TB, Streptococcus, Salmonella, Pseudomonas, Norovirus, RSV, H1N1, ESBL, CRKP and Rotavirus, Hepatitis B, Hepatitis C, Enterobacter cloacae NDM-1, Escherichia coli NDM-1, Klebsiella pneumoniae NDM-1, MDR Enterococcus faecium, MDR Staphylococcus aureus, VRSA; two-minute kill time for MRSA, VRE, Staph; and a four-minute kill time for C. difficile spores.
The individually packaged towels are saturated with the 1:10 dilution of sodium hypochlorite and are stabilized with detergent for a long shelf life. Save time and labor of mixing in-house bleach while ensuring disinfectant is efficacious. HYPE-WIPES are portable, convenient and safe for disinfecting hard, non-porous surfaces at clinical and research labs, physician and dental offices, ambulances, waste-water facilities, and blood banks. Available in two sizes: Full (6” x 12”) and Mini ( 3” x 3”). Current Technologies
The MicroFlow III is a Class 1 ductless carbon filtered workstation equipped with Activated Carbon filtration ideal for fumes, odors, and non-hazardous chemical vapors. Dimensions are 24” wide X 20.75” high X 24” deep. Completely self contained with integral recessed work surface to contain spills. A convenient clear viewing sash surrounds the work area for user protection. Sash can be conformed for use with a microscope and is easily removable. Variable speed fan control allows for high speed 100f/m. air flow through the sash opening, or medium and low flow for sensitive operations.
Typical applications include, sample weighing, general chemistry involving small volumes of common chemicals, individual work stations, tissue staining and processing, gluing and drying operations, solvent cleaning of electronic parts, soldering fumes and odors, school demonstration workstations, containment of forensic applications. HEMCO
INTEGRA offers an extensive range of manual and electronic handheld pipettes –
combined with the GripTips system – they help take the ‘pain’ out of multichannel
pipetting, boosting productivity.
The difficulties associated with using handheld multichannel pipettes are familiar to every lab scientist; tips need ‘hammering’ on to ensure they are picked up, but this still doesn’t always ensure correct tip alignment or a good seal, potentially affecting assay results. Add to this the extra force required to eject eight, 12 or even 16 tips at a time and the pain of using multichannel pipettes becomes all too real.
INTEGRA’s handheld multichannel pipettes and GripTip system have been developed to eliminate these issues, allowing tips to effortlessly snap onto the pipette and ensuring they are always firmly attached, perfectly aligned and easily ejected. Integra
A new Insight CMOS Camera featuring Sony’s Pregius sensor offers a generation of cameras that delivers features previously found in much higher-priced cameras. The camera’s USB 3.0 interface and global shutter work with a SPOT software auto-exposure scheme produce distortion-free, live image viewing of moving specimens at 22 fps, at full resolution. The combined low read-noise and high QE, furnishes sensitivity not previously seen in uncooled cameras.
The wide range of capabilities means many applications are supported by the Insight sCMOS cameras including live-cell, brightfield, histology, pathology, cytology and bright
fluorescence imaging. Spot Imaging
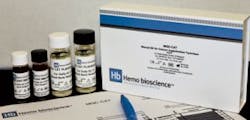
Hemo Bioscience announced that MQC-CAT, the first and only “ready to use” quality control product for manual column agglutination technology (CAT) blood bank testing methods, has received FDA 510(k) clearance. Validated for use in all manual CAT testing systems currently available in the US, MQCCAT includes both positive and negative red cells and serums to ensure that ABO and Rh(D) as well as antibody screening and identification reagents are performing as expected. Hemo Bioscience