Laboratory results, including immunoassay results, influence an estimated 70 percent of healthcare decisions. When healthcare decisions are based on erroneous results due to biotin interference, there is a risk of inappropriate and sometimes irreversible treatment.
Biotin interference has been a risk since streptavidin-biotin methods first began to be used in immunoassays decades ago. Last year, an atypically high number of reports documenting false laboratory results due to biotin interference were published. In the same year, laboratory professionals received an urgent field safety notice related to more than 20 lot numbers of a troponin I immunoassay which showed >10 percent change in results for samples with biotin levels ≤10 ng/mL; this was inconsistent with the product’s instructions for use.1
Recently, a patient consuming over-the-counter (OTC) biotin supplements was inappropriately scheduled for radioiodine thyroid ablation due to erroneous thyroid results. This potential patient safety issue was averted due to the extra laboratory testing and diligent communication with the clinician.2
To successfully manage risks associated with biotin interference, clinical laboratory professionals can benefit from a better understanding of vitamin supplementation trends, therapeutic uses of biotin, the prevalence of biotin-based immunoassays, immunoassay designs, biotin interference mechanisms, and strategies to mitigate this inference risk (such as the use biotin-free assays).
Biotin trends and therapy
More than 66 percent of adults in the United States take dietary supplements, which can include biotin,3 an essential vitamin which enhances skin, nerve, and digestive system health as well as embryonic development. This vitamin also supports energy release and the metabolism of proteins, carbohydrates, and fats. Biotin deficiency can lead to neurological disease and affect the growth of hair and skin cells.
Many OTC supplements contain biotin megadoses, up to 10 mg per pill, which is more than 300 times the recommended daily intake.4 Consumption of a 1.5 mg dose of biotin has been linked to an unexpectedly low parathyroid hormone (PTH) level.5 Healthy study participants who consumed 15 mg to 30 mg of biotin daily were shown to have biotin concentrations of over 30 ng/mL; these levels could interfere with biotin-based immunoassays.6
Among the users of biotin supplements today are pregnant and lactating women, some of whom consume daily supplements containing the vitamin. Patients are often unaware that some multi-vitamins contain biotin, and their medical history may not note their OTC biotin intake. Biotin is alternately known as vitamin H or B7, potentially further contributing to patients’ confusion regarding their biotin intake status.
Biotin treatment may benefit patients with conditions such as multiple sclerosis, inherited metabolic diseases, and diabetes mellitus.7 Patients who have clinical symptoms of dietary biotin deficiency such as skin rash and hair loss may also benefit from biotin doses. Individuals on hemodialysis or peritoneal dialysis may be prescribed biotin as medical therapy.
Biotin-based immunoassays
Biotin-based methods are used in popular immunoassay designs because these methods provide a convenient and efficient mechanism to accurately separate immobilized antigen from free antigen.6 The biotin molecule, which binds irreversibly to avidin, can be conjugated to an immunoglobulin molecule by a straightforward amino substitution at an alkaline pH with no loss of immunoglobulin activity. Streptavidin-biotin assays, containing a protein similar to avidin, found in Streptomyces avidinii, are now widely used in several immunoassay platforms .6,8,9
Most automated immunoassays are based on two major assay designs: the sandwich format and the competitive format. Both formats generally incorporate a method to separate immune complexes on a solid phase such as a magnetic bead or particle. These two assay formats can be biotin-free (Figures 1, 2), or these assays can utilize the streptavidin-biotin interaction. (Figures 3, 4)
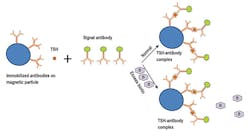
Sandwich assays are used to measure large molecules such PTH and thyroid stimulating hormone (TSH). For example, TSH is “sandwiched” between two different anti-TSH antibodies. One of these antibodies is labeled with signal, typically a fluorescent or chemiluminescent compound that is measured by an instrument. The other “capture” antibody allows the immobilization and separation of the immune complexes on a magnetic particle. The signal measured is directly proportional to the concentration of an analyte such as TSH. (Figure 1)
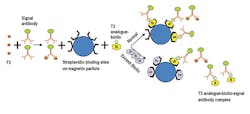
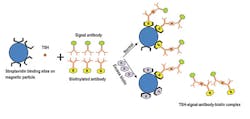
Biotin-based sandwich assays may include streptavidin bound to the magnetic particles and biotinylated antibodies. TSH is sandwiched between the biotinylated capture antibody bound to streptavidin and the signal antibody. As with the biotin-free sandwich assay, a higher signal is interpreted as a higher level of TSH. (Figure 3)
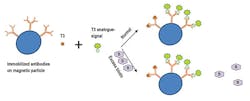
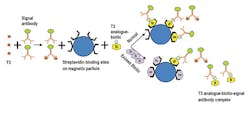
Competitive assays are used to measure molecules that are too small to allow the simultaneous binding of two antibodies without steric hindrance. These small molecules include triiodothyronine (T3) and thyroxine (T4). Using T3 as an example, magnetic particles are coated with anti-T3 capture antibodies, and the particles are added to a reaction mixture containing endogenous T3 in the patient’s sample and analogue T3 labeled with signal. The endogenous T3 competes with the labeled analogue T3 for capture antibody binding sites. In contrast to sandwich assays, the signal is inversely proportional to the concentration of the analyte such as T3. (Figure 2)
In biotin-based competitive assays, a typical assay format may include endogenous T3 being combined with an excess amount of signal antibody, resulting in a competition for signal antibody binding sites. Some signal antibody binding sites will be bound to endogenous T3 and other signal antibodies will not be bound to T3. The unbound signal antibodies bind to biotinylated T3 analogues, resulting in biotinylated analogue-signal antibody complexes. These biotinylated analogue-signal antibody complexes attach to the biotin binding sites of streptavidin-coated magnetic particles and become immobilized. As noted for biotin-free competitive assays, a higher signal indicates a lower T3 concentration in the
patient’s sample. (Figure 4)
Biotin interference mechanisms
Biotin-based immunoassays are exposed to interference risk because endogenous biotin from supplement intake can compete with biotinylated antibodies or biotinylated analyte analogues for binding sites on streptavidin attached to magnetic particles. Some immunoassays like 25-OH Vitamin D are more susceptible to biotin inference than other assays.6
In sandwich immunoassays, endogenous biotin binding to streptavidin sites can block the analyte-antibody-biotin complexes from binding and becoming immobilized. (Figure 3) This may lead to a very low signal that can be misinterpreted as a low result. In competitive assays, endogenous biotin can block biotinylated analogue-signal antibody complexes from binding to streptavidin attached to magnetic particles. (Figure 4) This could lead to a very low signal that could be misinterpreted as a high result.
Risk mitigation strategies
Laboratory professionals can implement strategic risk mitigation associated with biotin interference. One strategy may involve close collaboration between the laboratory and the clinical staff to identify biotin-consuming patients. Such patients may need to delay blood specimen collection for several days after biotin cessation, depending on their dose and their prescribed immunoassay(s).10 Another strategy could be to strengthen test result review procedures that are designed to detect false results associated with suspected assay interferences. For example, laboratory management can tighten the criteria requiring follow-up investigation when results are inconsistent with the clinical profile. (This strategy can lead to delays in reporting results and the need for clear criteria applied to complex clinical profiles.) Finally, a strategic shift to biotin-free immunoassays would eliminate risk associated with biotin interference.
The increasing consumption of biotin megadoses have heightened the risk of laboratories reporting erroneous results that could lead to misdiagnosis. At the decision cut-off value, even very low interference may result in misdiagnosis and possible risk to patient safety. The vitamin megatrend seems likely to continue; perhaps the only definitive mitigation strategy for biotin interference risk is use of biotin-free immunoassay platforms.
REFERENCES
- Health Canada, “Recall and safety alerts Canada,” Government of Canada, 25 May 2016. http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2016/58522r-eng.php
- Katzman BM, Rosemark C, Hendrix BK, et al. Investigation of biotin interference in common thyroid function tests using the Roche Elecsys® system. Clin Chem. 2016;62(S10):S75
- Moloughney S. Positioning the supplement market for long‐term success. Nutraceuticals World. 2016. http://www.nutraceuticalsworld.com/issues/2016-04/view_features/positioning-the-supplement-market-for-long-term-success/
- Paxton A. Beauty fad’s ugly downside: test interference. CAP Today Online. 2016.http://www.captodayonline.com/beauty‐fads‐ugly‐downsidetest‐interference/
- Waghray A, Milas M, Nyalakonda K, Siperstein AE. Falsely low parathyroid hormone secondary to biotin interference: a case series. Endocrine Practice. 2013;19(3)1-14.
- Clerico A, Plebani M. Biotin interference on immunoassay methods: sporadic cases or hidden epidemic? Clin Chem Lab Med. 2017.
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ Disease. NEJM 2016;375:1098-1099
- Christopoulos E, Diamandis EP. The biotin‐(strept)avidin system: principles and applications in biotechnology. Clin Chem. 1991;37(5):625-636.
- Holmes E.W. et al. Biotin supplements: a hair‐raising challenge to the analytical accuracy of clinical immunoassays. The FASEB Journal. 2017;31(Supplement 1):795.
- Piketty ML, Polak M, Flechtner I. et al. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clinical Chemistry and Laboratory Medicine. 2016.
Lori Lai, DHSc, serves as Senior CL-AIA Product Manager for Tosoh Bioscience, Inc., provider of AIA-PACK immunoassays and AIA Systems. She was introduced to streptavidin-biotin methods working with another firm in the industry, after an early career as a California licensed medical technologist.