Identifying and managing hemolysis interference with CBC specimens
Q
Can you inform me of an efficient way to detect and handle hemolysis in a CBC specimen? We need to address CAP HEM.22200. Currently we do not have a great procedure for this item in our checklist.
A
Hemolysis is defined as the release of hemoglobin and other intracellular components as a result of red blood cell (RBC) destruction. Specifically, hemolysis is present if the free hemoglobin is greater than 0.3 g/L.1 The effect on the complete blood count (CBC) results due to red cell destruction inaccurately decreases the red blood cell (RBC) count and the hematocrit (when calculated), while the hemoglobin (Hgb) and MCV values remain the same. The platelet count may also be inaccurate due to RBC fragments.
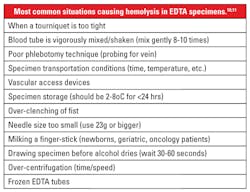
Hemolysis may occur in vivo due to metabolic disorders (such as hemolytic disease of the newborn and autoimmune hemolytic anemia), chemicals or drugs, mechanical devices, and infectious agents. In vitro hemolysis most often occurs as a result of poor pre-analytic practices. (Table 1).1,2
Hemolysis is one of the most frequent reasons why specimens are rejected for analysis.1,2 While only 3.3 percent of all blood specimens received in the laboratory may be hemolyzed, such specimens make up almost one-half to three-quarters of specimens that are unacceptable for analysis. A CAP Survey reported that more than two-thirds of participating laboratories had less than three percent of hemolyzed specimens, whereas one-fifth reported up to 15 percent or more hemolyzed specimens.3
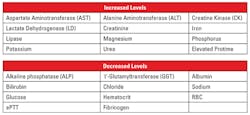
In addition to the release of hemoglobin, erythrocytic structural proteins, enzymes, lipids, and carbohydrates may also be present, causing interference with other laboratory assays besides the CBC.1,4 A number of analytes may be affected by hemolysis; however, potassium and lactate dehydrogenase (LD) levels can be 20 and 150 times higher than that seen in serum levels, respectively (Table 2).2
So, how to prevent hemolysis—one of the most frequently interfering occurrences that is observed in the clinical laboratory?
Routine visual identification for hemolysis in laboratory samples has been shown to be inconsistent and lacking reproducibility among staff observers. Detecting hemolysis is further complicated if bilirubin (icterus) is present, which is an especially significant problem with newborn specimens. While some chemistry analyzers can provide serum indices (Hemolytic Index) that can assist in detecting hemolysis, hematology analyzers have not, to-date, been adapted to perform this task.2,3,5
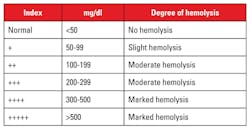
Detecting hemolysis in hematology specimens on a routine basis is challenging. To visually detect hemolysis in CBC specimens, the EDTA tubes would have to sit for a period of time in order to allow the natural separation of red cells from the plasma. But as noted, variation and proper detection of hemolysis by visual inspection can be subjective, even though comparison charts are available.6 Table 3 shows comparative levels of hemolysis as it relates to the amount of hemoglobin present.
Each laboratory should have an appropriately approved policy with respect to handling specimens that are hemolyzed, lipemic, and/or icteric. The best approach to identifying interfering substances with laboratory specimens is to establish pre-analytic protocols in specimen procurement, transportation, and storage. Staff training is critical to successfully implement these protocols.
Further, preventive measures besides good staff training would include establishing a quality assurance program with appropriate indicators, such as those suggested by CAP’s Q-Tracks.7 Follow-up includes ongoing monitoring for potential weak areas and implementing corrective actions as needed, documenting the entire process. Reviewing Table 1 and the various situations that may cause hemolysis might suggest areas to monitor.
At the analytical phase of testing, review (manually or through auto-verification) of each processed CBC for unlikely test values can also tip-off a situation involving an interfering substance. Review of the mean corpuscular hemoglobin (MCH = [Hgbx10]/RBC]) and the mean corpuscular hemoglobin concentration (MCHC = [Hgbx100]/Hct) values would alert staff of potentially unexpected parameters requiring further inspection. Since hemolysis has little effect on the MCV or the hemoglobin results, the RBC count and hematocrit values would be decreased, thus falsely altering the calculations for the MCH and MCHC.
One additional opportunity to identify hemolysis is review of delta checks. MCV is one of the most consistent CBC values for each patient, changing only with the presence of an acquired disorder (various anemias) or transfusion. It is also the most often used delta check.8 While not all patients are going to have multiple CBCs performed within a given period, it still may serve as another opportunity to catch compromised specimens. Another indicator would be a significant increase in LD and/or K levels when chemistry tests are obtained with the same draw as the CBC and are available.
Another issue to consider is the reporting of laboratory values in the presence of hemolysis—what should be reported, and how it should be reported. A protocol addressing these issues as well as who authorizes release of the information is essential. Prompt notification to the patient’s healthcare practitioner of the situation is also important in order to determine if a repeat specimen needs to be procured.
While not all patient specimens will allow for extensive instigative opportunities, raising the level of awareness of these potential issues is a good first step by incorporating appropriate protocols and documentation. Ensuring that an active quality management program is in place with documentation and constant follow-up activities can go a long way in addressing inspection protocols.
REFERENCES
- Lippi G, Blanckaert N, Bonini P, et al. Haemolysis: An overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med. 2008;46(6):764-772.
- Katzman B, Baumann N. Detecting and handling hemolysis using serum indices. Clin Lab News, AACC. March 2016. https://www.aacc.org/publications/cln/articles/2016/march/detecting-and-handling-hemolysis-using-serum-indices.
- Lippi G, Montagnana M, Salvagno GL, Guidi GC. Interference of blood cell lysis on routine coagulation testing. Arch Pathol Lab Med. 2006;130(2):181-184.
- Simundic AM, Topic E, Nikolac N, Lippi G. Hemolysis detection and management of hemolyzed specimens. Biochemia Medica. 2010;20(2):154-159.
- Howanitz PJ, Lehman CM, Jones BA, et al. Practices for identifying and rejecting hemolyzed specimens are highly variable in clinical laboratories. Arch Pathol Lab Med. 2015; 139(8):1014-1019.
- Hemolysis Comparison Charts.
- Ford A. Delta checks as a safety net: how used, how useful? CAP Today. 2015;29(9):22-24.
- Nakhleh RE, Souers RJ, Bashleben CP, et al. Fifteen years’ experience of a College of American Pathologists program for continuous monitoring and improvement. Arch of Pathol Lab Med. 2014;138(9):1150-1155.
- Adiga U, Yogish S. Hemolytic index—a tool to measure hemolysis in vitro. IOSR J Biotechnol Biochem. 2016;2(2):49-52.
- Lippi G, Blanckaert N. In vitro hemolysis: Causes, prevalence, effects, measurements & solutions. Presentation: EuroMedLab 2009, Innsbruck, ISW #20. June 2009. http://www.specimencare.com/resource.aspx?IDX=11331.
- Arzoumanian L. Tech Talk. 2003;2(2). http://www.bd.com/vacutainer/faqs/.
About the Author

Anthony Kurec, MS, MASCP, MLT, H(ASCP)DLM
is Clinical Associate Professor, Emeritus, at SUNY Upstate Medical University in Syracuse, NY. He is also a member of the MLO Editorial Advisory Board.