ALCOR Scientific’s iSED is a fully automated ESR analyzer which utilizes EDTA blood samples directly from the primary collection tube. The test is performed utilizing 100 microliters of blood, and the results are obtained in only 20 seconds after automatic mixing. The iSED is ideal for pediatric testing because it requires only 100µl of blood and virtually eliminates QNS issues. The BD Microtainer MAP Microtube is fully compatible with the iSED without any additional sample manipulation.
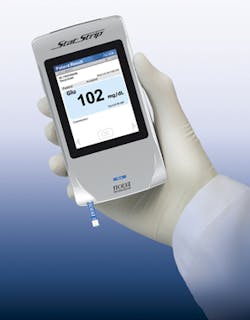
Nova Biomedical’s StatStrip Glucose hospital meter system is a blood glucose monitoring system that is FDA-cleared for use in all healthcare settings, including intensive care. The StatStrip Glucose is used for the detection and management of dysglycemia. It is also CLIA-waived in all settings. The system measures and corrects for interferences such as hematocrit, ascorbic acid, uric acid, and other electrochemical interferences. StatStrip has no interference from oxygen, pH, sodium, or non-glucose sugars, including maltose and galactose. Results are available in only six seconds, using 1.2 microliters of whole blood.
Milestone introduces SealSAFE, a system for eliminating formalin exposure and standardizing fixative volumes during specimen management and transport. SealSAFE enables surgical and laboratory professionals to safely manage and transfer patient specimens from the OR to pathology using laboratory grade vacuum bags. Specimen bags are placed into a sealing chamber and automatically weighed; then a preselected ratio of fixitive is added prior to vacuum completion. The fully vented unit extracts formalin fumes from the reagent drawer and sealing chamber, keeping users safe. Benefits include reduced exposure to harmful fumes, lower fixative use through more efficient ratios, documentation of fixation start time, barcode identification of specimens, and cost reductions in waste disposal of archived case tissue.
Mindray BS-480 chemistry analyzer is a versatile mid- to high-volume chemistry platform with conventional software design and easy-to-navigate interface. The wide selection test menu includes general chemistries, electrolytes, and drugs-of-abuse testing which serves the needs of most clinical laboratories and hospitals. Features of the analyzer include: constant 400 T/H, up to 560 T/H with ISE; 80 reagent positions; 90 sample positions; 90 permanent glass cuvettes; 8-steps auto-washing. The BS-480 has a grating optical system: 340nm to 800nm; R1, R2, R3, R4 reagent combo; and probe level sense, collision, bubble and clot detection. The device has auto-rerun, auto-serum index, auto-dilution; and auto-reflexive testing. It has a
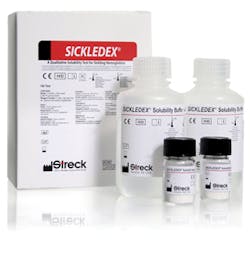
Streck’s SICKLEDEX is a qualitative solubility test kit used to detect the presence of sickling hemoglobin in human blood or sickle cell control material. Kits are conveniently packaged with two separate squeeze bottles of phosphate buffer with Saponin for easy dispensing, and to eliminate waste. The kits also contain two buffer dispensing caps and two vials of Sodium Hydrosulfite reagent powder. Each test requires 2ml of reconstituted solubility buffer and 20µl of anticoagulated whole blood or 20µl of sickle cell hemoglobin control. A 10-place test tube reading rack is also available.
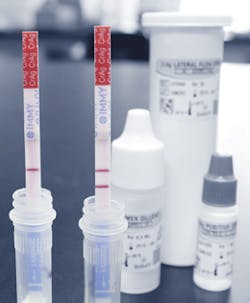
The IMMY CrAg LFA (Cryptococcal Antigen Lateral Flow Assay) is an immunochromatographic dipstick assay for the qualitative and semiquanitative detection of cryptococcal antigen. This lateral flow assay delivers analytical sensitivity that is up to 200x more sensitive than other commercial assays. The CrAg LFA assists healthcare providers in all clinical settings with rapid, reliable, and robust diagnostic results. The CrAg LFA is FDA-cleared for both C. neoformans and C. gattii, providing sensitivity across all four serotypes of Cryptococcus. With a 2-year shelf life, room temperature storage, and moderate complexity CLIA rating, the CrAg LFA can be offered on all laboratory shifts, on a STAT basis, and as a point-of-care assay.