ASM offers “enhanced precautions” for handling specimens from suspected Ebola patients
On August 26, 2014, the Centers for Disease Control and Prevention (CDC) provided an updated interim guidance for collecting, transporting, and testing specimens from persons under investigation for Ebola virus disease (www.cdc.gov/vhf/ebola/hcp/interim-guidance-specimen-collection-submission-patients-suspected-infection-ebola.html.). The CDC states that U.S. clinical laboratories can safely handle specimens from these potential Ebola patients by taking all required precautions and practices in the laboratory, specifically designed for pathogens spread in the blood; that risk assessments should be conducted by each laboratory director, biosafety officer, or other responsible person to determine the potential for sprays, splashes, or aerosol generated during laboratory procedures; and that any person collecting specimens from a patient with suspected Ebola virus disease should wear gloves, water-resistant gowns, full face shield or goggles, and masks to cover all of nose and mouth.
The CDC further states that any person testing specimens from a patient with a suspected case of Ebola virus disease should wear gloves, water-resistant gowns, full face shield or goggles, and masks to cover all of nose and mouth, and as an added precaution use a certified class II Biosafety cabinet (BSC) or plastic splash guard with PPE to protect skin and mucous membranes. In addition, the agency states that all manufacturer-installed safety features for laboratory instruments should be used. The CDC indicates that routine laboratory testing including traditional chemistry, hematology, and other laboratory testing can be used to support and treat patients, and that the precautions as described offer appropriate protection for healthcare personnel performing laboratory testing on specimens from patients with suspected infection with Ebola virus.
The American Society for Microbiology (ASM) Committee on Laboratory Practices has also released guidance, dated September 10, 2014 (www.asm.org/images/PSAB/Ebola9-10-14.pdf.). In this guidance, the ASM indicates that it agrees with the CDC recommendations that specimens from suspect HFV (Hemorrhagic Fever Virus) patients may arrive at the routine testing laboratory without the knowledge of the laboratory and that all laboratory testing must follow standard precautions. In addition, the ASM has drafted a document outlining “enhanced precautions” which some institutions may choose to adopt in order to assure the safety of their testing personnel and to provide appropriate medically necessary laboratory testing to suspect HFV patients. The ASM document is presented as one possible approach to testing of patients with suspected HFV. ASM further provides that any guidelines for testing should be thoroughly discussed with the appropriate medical personnel prior to implementation and may include significant modifications of their recommendations. These recommendations also give detailed descriptions for blood culture and malaria testing.
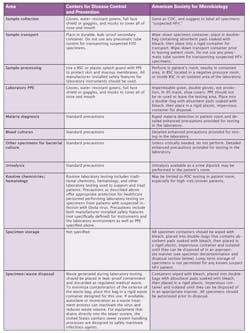
Table 1 compares and contrasts the CDC and the ASM recommendations with regard to laboratory specimen testing of suspect Ebola Viral Disease-EVD/Hemorrhagic Fever Virus-HFV patients.
Until Ebola infections are brought under control in West Africa, U.S. medical facilities may see patients at risk for this infection. Both the CDC and the ASM have drafted guidance for laboratory testing of suspect patients; these guidelines have some similarities and significant differences. The CDC guidance relies heavily on the use of standard precautions for laboratory testing while the ASM, due to our limited experience with this virus, the high case-fatality rate, the unparalleled West African outbreak, and the understandable concern on the part of the laboratory community, is providing guidance for enhanced precautions when dealing with such specimens. The ASM and CDC agree it is essential that laboratory staff review laboratory safety procedures and guidelines to ensure that protocols designed for bloodborne pathogens are followed, and that testing personnel are fully familiar with all additional safety protocols if medical facilities choose to incorporate enhanced safety precautions.
About the Author

Susan E. Sharp, PhD, DABMM, FAAM
Ph.D., DABMM, FAAM, serves as Scientific Director for Copan Diagnostics, Inc., U.S. Dr. Sharp’s most prominent area of interest has centered on cost-effective, clinically-relevant, diagnostic microbiology. Sharp has served as a director for microbiology laboratory services for over 30 years.