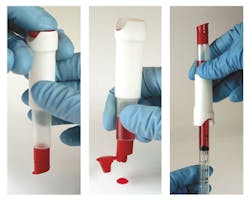
CueSee Glucose is a real blood control for validating precision and accuracy of glucose meters. Its real blood matrix makes it highly commutable and compatible with all glucose devices, exhibiting minimal matrix effects. The CueSee’s low imprecision makes it comparable with that of human whole blood. The ACU-Drop II is the packaging form key to delivering this control in a dual-chambered device, keeping the various fractions separated to prevent reactions between components of the desired matrix. Push a button to allow fractions to combine, mix, and the sample is ready to use either from the built-in dropper bottle or by attaching a syringe. Use for QC, calibration verification, proficiency testing, competency assessment, method validations, and lot comparisons.
The Nova StatStrip glucose monitoring system measures and corrects for interferences such as hematocrit, ascorbic acid, uric acid, and other electrochemical interferences. StatStrip has no interference from oxygen, pH, sodium, or non-glucose sugars, including maltose and galactose. Results are available in only six seconds, using 1.2 microliters of whole blood. Statstrip technology meets the escalating demands of hospital glucose testing. It elevates bedside glucose testing to a level of accuracy, precision, and patient safety that approaches the quality of central laboratory testing.
Streck’s A1c-Cellular is a whole red blood cell HbA1c control with intact red blood cells. The A1c-Cellular Linearity is commercially available HbA1c linearity/calibration verification material with intact red blood cells. Both products test the entire HbA1c procedure, including the lysing of the red blood cells. Available in plastic cap-pierceable vials, which allow autosampling for HbA1c analyzers with that capability.
Siemens’ DCA Vantage Analyzer is a point of care immunoassay analyzer designed to monitor glycemic control and detect early kidney disease in diabetic patients within decentralized settings. Glycemic control is monitored through the use of HbA1c test results from a small whole blood sample in approximately six minutes. HbA1c patient trending graphs are also used and can be viewed or printed. Capabilities for detecting early kidney disease in patients include the use of albumin, creatinine, and albumin-to-creatinine ratio results from urine samples. In addition, an onboard GFR calculator indexes kidney function. With POCT-A2 communication protocol, the DCA Vantage Analyzer offers connectivity to LIS/HIS, RapidComm System, or other third-party POC data management systems.
The Randox Fructosamine assay offers a high quality enzymatic methodology for the measurement of fructosamine in serum or plasma samples. The assay is presented in a liquid ready-to-use format and has a measuring range of 8.12-1803 µmol/l. A wide range of instrument specific applications are available, making the Randox Fructosamine assay suitable for use on a variety of clinical chemistry analyzers. Complementary fructosamine controls and calibrator are also available from Randox, delivering a complete testing package.
Roche’s Tina-quant HbA1cDx Gen. 2 assay is a HbA1c test to aid in the diagnosis of diabetes. This test is available for the COBAS INTEGRA 800 platform. The Tina-quant assay meets specific performance criteria that make identifying and managing individuals with diabetes mellitus easier. Due to the high specificity of the reagent, this test is not affected by interferences from common hemoglobin variants such as HbS, HbE, HbC, and HbD. The Tina-quant HbA1cDx Gen.2 test delivers accurate results that help healthcare professionals manage patients reliably. The reporting in IFCC units (mmol/mol) and derived NGSP units (% HbA1c) eliminates any confusion for the healthcare professional. This test is also used as a reference method in NGSP Network Laboratories, an association that favors the standardization of HbA1c testing.
The LiquiColor ß-Hydroxybutyrate chemistry reagent (from Stanbio Laboratory, an EKF Diagnostics Company) is an enzymatic assay used by many healthcare systems. The assay runs on an open channel of a laboratory analyzer. The reagent can quantifiably detect the presence of ketones in patients with suspected diabetic ketoacidosis. It provides highly sensitive and precise results for the β-HB ketone that can be captured in an LIS system for easy reference. This assay can provide earlier detection for clinically significant ketosis and improve ER and CDU throughput. Controls and linearity standards are available and all reagents have a 24-month shelf life from date of manufacture.
The Tosoh G8 (Glycohemoglobin Analyzer HLC-723G8) has been cleared by the FDA as a HPLC instrument to be used as an aid in the diagnosis of diabetes and identifying patients who may be at risk for developing diabetes. The G8 utilizes gold standard ion-exchange HPLC with visual identification of hemoglobinopathies. The G8 is designed from the user perspective and features flag level sophistication to ensure only the highest quality HbA1c results are reported. The G8 has a compact footprint, analysis time of 1.6 minutes, and simple touch-screen operation. With no sample pre-treatment and tubes that can be continuous sample loading, the G8 easily adapts to changing laboratory workloads with either a 90 sample loader or a 290 sample loader.