Improving patient outcomes: State-of-the-art allergy and autoimmune diagnostic testing
Laboratory test results influence approximately 80% of all healthcare decisions,1 and as healthcare reimbursement moves from fee-for-service to measurable outcomes, accurate and cost-efficient diagnostic testing will only become more important. Guideline-based allergy and immunodiagnostic testing2,3 can enhance patient outcomes by providing accurate, consistent test results to confirm diagnoses and inform treatment decisions.4 With more than 40 years of technological advances in allergy and autoimmune diagnostics behind them, reliable and informative assays and fully automated instrumentation offer cutting-edge capabilities in the laboratory that can enhance clinical practice.
Allergy testing informs primary care and specialist practice
In the United States, suspected and diagnosed airborne allergies account for 28.4 million ambulatory visits and several hundred deaths annually.5,6 The National Institutes of Health (NIH) Guidelines for the Diagnosis and Management of Asthma2 and Guidelines for the Diagnosis and Management of Food Allergy4 support the use of specific IgE (s-IgE) blood testing, along with a detailed history and physical examination, to document an allergy diagnosis. Approximately 80% of patients with allergy-like symptoms and an estimated 76% of asthma patients are managed by primary care physicians.7 According to Allergies Across America,8 the widespread availability of blood-based s-IgE testing is changing clinical practice as these physicians order tests earlier, confirm a diagnosis, and institute targeted therapy. Referrals to allergy specialists can be made in cases of diagnostic uncertainty, uncontrolled symptoms, or evaluation for specific immunotherapy (SIT). In every region where s-IgE blood testing has increased among primary care physicians, so have referrals to allergy specialists.
Over decades of experience, increasingly sophisticated s-IgE testing has linked quantitative results with clinical symptoms and outcomes in empirical studies. For example, clinical decision points have been set for s-IgE antibodies to egg, milk, fish, and peanut and can be used to predict the probability that a child will react to these common food allergens.9-11 And with aeroallergens, s-IgE levels at age three years can be used to predict the likelihood of wheeze at age five years.12,13 One challenge for laboratory directors as well as physicians is that results from the three in vitro s-IgE testing methods approved by the Food and Drug Administration (FDA) are not interchangeable, although all are quantitative and express results in kilounits per liter. Results from the various methods are likely to be similar, but physicians should know that interpretive cut-offs from one method cannot be generalized to others.14
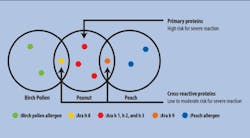
Two recent developments have further increased the clinical utility of s-IgE testing. The first is component-resolved diagnostic testing, which identifies IgE sensitization to specific allergenic proteins, not just to a whole-source extract containing multiple potential allergens.15 This testing quantifies s-IgE antibodies to primary, species-specific allergen components as well as to cross-reactive proteins. In the case of peanut, for example, component resolved diagnostics assess the patient’s clinical risk for a systemic allergic reaction.16 Sensitization to species-specific components (such as Ara h 1, 2, and 3 in peanut) indicates that the food source is the primary cause of clinical symptoms. Cross-reactive components are conserved across the plant kingdom, and s-IgE sensitization can also occur to a structurally homologous component from another source (Ara h 8 or 9, or a cross-reactive carbohydrate determinant [CCD] in peanut).17 Patients sensitized to peanut-specific Ara h 1, 2, or 3 are at high risk for systemic and severe reactions to peanut, whereas patients sensitized to other components of peanut face less risk (Figure 1).18 Such information is especially helpful when primary care providers see patients who have positive s-IgE results to whole-source extracts but ambiguous histories and unknown risk. Based on component-resolved diagnostic results, an allergy specialist can determine whether an oral food challenge is appropriate, or whether symptoms will likely be mild or localized, as in oral allergy syndrome.
The second development is a highly advanced biochip microarray used to determine a patient’s s-IgE antibody phenotype.14 Using a 30 µL blood sample, this microscope sized slide immunoassay platform simultaneously detects 112 components representing 51 allergen sources. The semi-quantitative results paint a detailed portrait of a patient’s allergic sensitizations. These much more detailed data are being compared to laboratory and clinical studies performed over decades with whole-source allergens. As additional component testing data accumulate, the results can be related to clinical symptoms and outcomes, yielding the comparable predictive information available for existing s-IgE assays.
Autoimmune biomarkers clarify difficult diagnoses
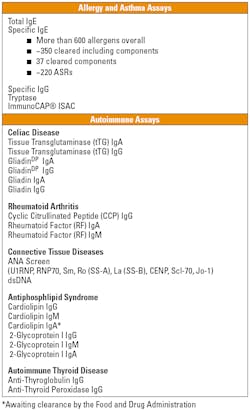
Autoimmune diseases affect more than 23.5 million Americans,19 yet diagnosing diseases such as celiac disease and rheumatoid arthritis (RA) has always been challenging. Widely varying and nonspecific symptoms, as well as genetic predisposition and the diversity of initiating agents, complicate the clinical diagnoses. However, various autoimmune diseases create detectable IgG, IgA, and IgM autoantibodies that can serve as biomarkers. Human recombinant antigens produce standardized and reproducible results for celiac disease, RA, connective tissue diseases, antiphospholipid syndrome, and autoimmune thyroid disease (Table 1).
Celiac Disease. The first serologic IgA screening test for celiac disease—the endomysial antibody or EMA assay—was introduced in 1983.20 In 1997 the antigen detected by EMA was identified as tissue transglutaminase (tTG), and today fully quantitative, highly automated recombinant human tTG assays are available (rhtTG). Patients with an IgA deficiency, which is 10 to 15 times more common in people with celiac disease than in those without, should be screened with a tTG IgG assay.21 The most recent European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines recommend using serologic assays before ordering an intestinal biopsy.22 Several recent studies suggest that biopsy might be avoided altogether when diagnosing celiac disease in pediatric patients with tTG IgA levels >10 X ULN.23,24
Rheumatoid Arthritis. In RA, joint destruction progresses rapidly if the disease is not diagnosed and aggressively treated in its early stages. Tests for rheumatoid factor (RF) proved of limited value because RF is not highly specific for RA. Antibodies to perinuclear factor (APF), keratin (AKA) and filaggrin were more specific to RA. Then, in 2000, Schelleken and colleagues demonstrated that cyclic citrullinated peptides (CCP) are the markers observed in RA,25 a discovery that led to the development of several anti-CCP antibody assays.26
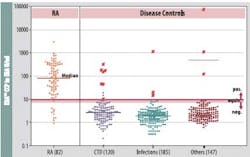
One commercially available anti-CCP assay possesses 96.7% specificity and 87.8% sensitivity and enables an early diagnosis.27 The assay performs exceptionally well when differentiating RA from other connective tissue diseases, such as systemic lupus erythematosis, Sjögren’s disease, and osteoarthritis (Figure 2). High specificity in routine clinical practice was confirmed in an Austrian study where the specificity was 97% (vs. 96.9% for ELISA); sensitivity was 80.3%, much greater than for ELISA (68.2%).28
A fully automated platform for allergy and autoimmune assays
Technologically refined laboratory systems have generally replaced labor-intensive, semi-qualitative testing for allergy and autoimmune diseases, while increasing output and decreasing turnaround time. Fully automated assay processing in one platform for both allergy and autoimmune diseases creates cost efficiency and flexibility in the laboratory. Technology based on one solid-phase anti-IgE binding method has become the standard for s-IgE allergy testing in comparative studies.29,30 According to global quality assessment programs, approximately 80% of laboratories worldwide employ this IgE assay.31 For autoimmune diseases, different tests can be combined in the same run, and no batching of samples is necessary, making small runs economically feasible. The systems require once-monthly calibration, with curve control applied to each run. First results are available in 100 minutes, then every 60 seconds, generating 350 results per shift. The high degree of automation entails minimal hands-on involvement and permits overnight runs. The Clinical Laboratory Improvement Act (CLIA) classifies the allergy and autoimmune diagnostic assays as having moderate complexity.
Laboratory directors can assist physicians in understanding various assay methodologies, ordering appropriate tests, and applying results. Continually evolving laboratory assays and equipment have streamlined the diagnosis of allergic and autoimmune diseases. Component-resolved diagnosis offers a more detailed analysis that can be used to determine a patient’s risk of experiencing a severe reaction. An advanced microarray simultaneously detects 112 allergenic components to yield a detailed portrait of a patient’s phenotype. Biomarkers for many autoimmune diseases are allowing earlier diagnosis and more timely and aggressive treatment. Efficient, fully automated laboratory systems can run both allergy and autoimmune assays with minimal supervision.
Four decades of diagnostic innovationThe discovery of IgE antibodies more than 40 years ago spurred the development of in vitro allergy tests that could detect circulating s-IgE antibodies in blood.32 The first commercially available s-IgE test was introduced in 1972. This semi-quantitative assay, called a radioallergosorbent test or RAST, measured bound s-IgE antibodies using allergens attached to paper disks. A modified RAST test, introduced in 1979, increased sensitivity by lowering cut-off values, doubling the sample volume, and lengthening incubation from three hours to overnight. Results from these early tests were considered unreliable because of the high number of false- negative results compared to skin prick testing, the sole method for determining allergic sensitization at the time. Current assays employ entirely different technology, and the American College of Allergy, Asthma and Immunology (ACAAI) and the American Academy of Allergy, Asthma and Immunology (AAAAI) maintain that it is no longer accurate to use RAST as a generic descriptor for s-IgE assays.33
New liquid and solid-phase assays that used monoclonal antibodies evolved in the 1980s and 1990s. Paper disks were replaced by cellulose sponges, polystyrene, or liquid-phase carriers that improved binding capacity. High-binding capacity is necessary for generating serum dilution curves that parallel the calibration curve. Today’s reference-standard s-IgE assay introduced a patented high-capacity “cap” design containing an allergen-coated, porous cellulose sponge with a large surface area where serum and allergen could bind to form antibody-antigen complexes. A robust covalent chemical bond attached allergens to the sponge, and mouse monoclonal anti-human IgE conjugates were added. After incubation, the sponge was vigorously washed to remove nonspecific IgE. The remaining complexes were first detected with radiolabeling, which has since been replaced with enzyme (fluorescent, colorimetric) or chemiluminescent labeling. This solid-phase technology provided greater sensitivity and true quantitative reporting of results in mass units of concentration.
The three s-IgE assays that predominate in the U.S. today differ in format, chemistries, reagent development, and analytic performance.34 All three assays demonstrate linearity between total s-IgE calibration curves and dilution of test sera, but results among assays differ by >20%. Thus, results from these assays are not interchangeable. Assays should conform to the World Health Organization 75/502 human IgE reference standard using a multipoint calibration curve for quantification, and testing should comply with the Clinical Laboratory Improvement Act of 1988, and with guidelines established by the Clinical Laboratory Standards Institute.35
Component resolved diagnostics extended allergy testing to single proteins purified from natural sources or produced using recombinant techniques. The first and only FDA-approved test for peanut components was launched in 2011. A panel of carefully selected components and whole-allergen extract allows detailed evaluation of peanut IgE sensitization patterns. The advantage of this approach lies in the ability to assess a patient’s clinical risk and institute appropriate dietary modifications based on that risk. Patients at low risk can be spared time-consuming and costly oral food challenges. Those at high risk must scrupulously avoid peanut in their diet.
The first allergy microarray features a fixed panel of 112 components that reside on the biochip. Antibodies from the serum sample bind to the allergen components, undergo a washing step, and then antibody complexes are detected by a fluorescence-labeled antibody. A biochip scanner equipped with proprietary software reports the semi-quantitative results in standardized units. The resulting phenotype profile can inform a diagnosis and help shape treatment plans.
About the Author
References
- Kuruvilla T. Where does the LIS go from here? MLO. 2011;43(8):26. https://www.mlo-online.com/articles/201108/where-does-the-LIS-go-from-here.php. Accessed February 21, 2013.
- National Institutes of Health. Guidelines for the Diagnosis and Management of Asthma. 2007. NIH publication 08-4051.
- Bizarro N, Tonutti E, Tozzoli R, Villalta D. Analytical and diagnostic characteristics of 11 2nd- and 3rd-generation immunoenzymatic methods for the detection of antibodies to citrullinated proteins. Clin Chem. 2007;53(8):1527-1533.
- National Institutes of Health. Guidelines for the Diagnosis and Management of Food Allergy in the United States. 2010. NIH publication 11-7700,
- CDC Fast Facts A-Z. Vital Health Statistics, 2003. Asthma and Allergy Foundation. Atlanta, GA: Centers for disease control; 2006.
- National Center for Health Statistics. Ambulatory Medical Care Utilization Estimates for 2007. Hyattsville, MD: NCHS; 2011. DHHS Pub. No. (PHS) 2011-1739, Series 13, No. 169.
- Duran-Tauleria E, Vignata G, Guedan MJ, Peterson CJ. The utility of specific immunoglobulin E measurements in primary care. Allergy. 2004;78:35-41.
- Quest Diagnostics. Allergies Across America. http://www.QuestDiagnostics.com/HealthTrends. Accessed January 15, 2012.
- Johansson SGO. ImmunoCAP specific IgE test: an objective tool for research and routine allergy diagnosis. Expert Rev Mol Diagn. 2004;4(3):273-279.
- Sampson HA. Food allergy—accurately identifying clinical reactivity. Allergy. 2005;60(suppl 79):19-24.
- Soderstrom L, Kober A, Ahlstedt S, et al. A further evaluation of the clinical use of sIgE antibody testing in allergic diseases. Allergy. 2003;58:921-928.
- Ahlstedt S, Murray CS. In vitro diagnosis of allergy: how to interpret IgE antibody results in clinical practice. Primary Care Respir J. 2006;15:288-236.
- Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol. 2005;116(4):744-749.
- Tufel G. Serology testing: striving for the gold standard. Clin Lab Prod. 2012;42:16-18.
- McClurkin C, Ortiz G. Utilizing molecular allergy for the diagnosis of peanut allergy. Adv Phy Asst. 2012;3(7):20-25.
- Borres MP, et al. Use of allergen components begins a new era in pediatric allergology. Pediatr Allergy Immunol. 2011;22(5):454-461.
- Hauser M, et al. Panallergens and their impact on the allergic patient. Allergy Asthma Clin Immunol. 2010;6(1):1.
- Nicolas N, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191-197.
- US Dept. of Health and Human Services. Office of Women’s Health. Autoimmune Diseases Fact Sheet. Washington, DC: DHHS; 2010. http://womenshealth.gov/publications/our-publications/fact-sheet/autoimmune-diseases.cfm#b. Accessed February 25, 2013.
- Reinhardt R, Yancon J. Persistent gastrointestinal distress—using serologic autoimmune biomarkers to clarify a diagnosis of celiac disease. MLO. 2012;45:8-12.
- Olsson NO, Musset L, Chyderiotis G, et al. Celiac disease. In: Shoenfeld Y, Meroni PL, eds. The General Practice Guide to Autoimmune Diseases. Miami, FL: Pabst Science Publishers; 2012: 193-201.
- Husby S, Koletzko S, Korponay-Szabó IR, et al. The ESPGHAN Working Group on Coeliac Disease Diagnosis 2011. ESPGHAN Guidelines for the diagnosis of coeliac disease in children and adolescents: an evidence-based approach. J Pediatr Gastroenterol Nutr. 2012; in press.
- Mubarak A, Wolters VM, Gerritsen AM, et al. A biopsy is not always necessary to diagnose celiac disease. J Pediatr Gastroenterol Nutr. 2011;52:554–557.
- Mubarak A, et al. Tissue transglutaminase above 100 U/ml and celiac disease: a prospective study. World J Gastroenterol. 2012;18(32):4399-4403.
- Schelleken GA, Visser H, de Jong BAW, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43(1):155-163.
- Correia ML, Carvalho S, Fortuna J, Pereira MH. Comparison of three anti-CCP antibody tests and rheumatoid factor in RA and control patients. Clin Rev Allergy Immunol. 2008,34:21-25.
- Thermo Fisher Scientific. EliA® CCP Directions for Use. http://www.phadia.com/PageFiles/29279/EliA%20CCP%20Well%20%284×12%29.pdf. Accessed Feb. 11, 2013.
- Herold M, Boeser V, Russe E, Klotz W. Anti-CCP: history and its usefulness. Clin Dev Immunol. 2005;12(2):131-135.
- Lockey R, Lichtenstein L, Bloch K, et al. Position statement: the use of in vitro tests for IgE antibody in the specific diagnosis of IgE-mediated disorders and in the formulation of allergen immunotherapy. J Allergy Clin Immunol. 2001;90:263-267.
- Dolen WK. Skin testing and immunoassays for allergen-specific IgE. Clin Rev Allergy Immunol. 2001;21:229-239.
- Killingsworth LM. Specific IgE testing: objective evidence of sensitization aids diagnosis and treatment decisions. Comp Ophthalmol Update. 2007;37(1):17-21.
- Ahlstedt S. Understanding the usefulness of specific IgE blood tests in allergy. Clin Exp All. 2002;32:11-16.
- Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
- Williams PB, Barnes JH, Szeinbach SI, et al. Analytic precision and accuracy of commercial immunoassays for specific IgE: establishing a standard. J Allergy Clin Immunol. 2000;105(6 part 1):1221-1230.
- Matsson P, Hamilton RG, Adkinson, et al. Evaluation methods and analytical performance characteristics of immunological assays for human immunoglobulin E (IgE) antibodies of defined allergen specificities. National Committee on Clinical Laboratory Standards (NCCLS).