Sensitivity and specificity of a new cryptococcal antigen lateral flow assay in serum and cerebrospinal fluid
Introduction
Cryptococcus neoformans and C. gattii are ubiquitous environmental pathogens and major causes of opportunistic infection in immunocompromised individuals. Among patients with HIV/AIDS, cryptococcal disease contributes to more than 600,000 deaths per year globally.1 Sub-Saharan Africa has the greatest burden of disease with more than 75% of the cases and 80% of the deaths due to cryptococcal disease worldwide.1 Furthermore, in the United States cryptococcal infection is increasingly recognized as a major cause of invasive fungal infection in solid organ transplant recipients. A recent review of more than 1,000 such patients identified cryptococcal infection as the third-most common fungal infection, following candida species and aspergillus species.2 The limited use of strategies to diagnose cryptococcal disease early, prior to the onset of meningitis, likely contributes to ongoing morbidity and mortality, particularly in low-resource settings.
Currently, the diagnostic tests most commonly used in the developed world for identifying cryptococcal antigen are the latex agglutination test (LA) and the enzyme immunoassay (EIA). In resource-limited settings, both are difficult to use, as they require refrigeration, pretreatment with additional enzymes such as pronase in the case of the LA, and specialized equipment such as a spectrophotometer in the case of the EIA.3 Use of the EIA often also results in a delayed turnaround time due to batch testing as well as the wastage of 96-well plates due to low sample volume.
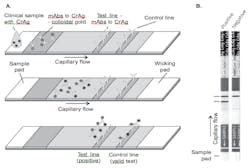
In 2009, a new lateral flow assay (LFA) was developed that enables the rapid detection of the cryptococcal polysaccharide capsule glucuoronoxylomannan. Similar to other point-of-care diagnostic tests, the LFA functions as a dipstick.4 Figure 1 illustrates a schematic showing operation of lateral flow immunochromatographic assay for the detection of cryptococcal antigen and images of positive and negative LFA. The assay utilizes monoclonal antibodies with broad reactivity across the four major cryptococcal serotypes (A and D for var neoformans, B and C for var gattii) and provides both qualitative and semi-quantitative results in ten minutes with no specimen pretreatment.4 Test materials do not require any refrigeration or cold-chain transport. Quantitative titers may be ascertained by mixing the specimen with diluent solution; the highest sample dilution that produces a positive result is considered the LFA titer.4
In July 2011, the Food and Drug Administration cleared the LFA for use in serum and cerebrospinal fluid (CSF) specimens based on a high level of agreement with the LA. We aimed to review the current available literature and data on the test performance characteristics of the cryptococcal antigen LFA in serum and CSF specimens.
Methods
We conducted a review of published literature and conference abstracts through August 21, 2012, to identify studies on the performance of the LFA in serum and CSF specimens. Searches were conducted on PubMed and Web of Science and were restricted to studies written in English. We used the keywords “cryptococcus” and “lateral flow assay” to define our search. Additional queries that included the keywords “diagnostic tests” and “cryptococcal antigen” did not yield any further information. Additional abstracts were obtained from specific conference meetings. We calculated the median (and range) of reported test performance values, percent sensitivity, and percent specificity.
Data regarding analytic sensitivity and specificity were obtained from the Food and Drug Administration submission file for the LFA. The analytic serotype sensitivities for the LFA, two currently available LAs, and an ELISA were compared using purified glucuronoxylomannan from multiple strains of four serotypes of Cryptococcus neoformans and C. gattii. Purified glucuronoxylomannan was run in each of the four assays at identical concentrations, following the manufacturer’s instructions for each assay.
The analytic specificity was measured by running clinical specimens with potentially cross-reacting pathogens such as Penicillium, Sporothrix, Blastomyces, Coccidioides, Histoplasma, and Candida as well as interferents such as rheumatoid factor, bilirubin, and lipoprotein. Cross-reactivity with Aspergillus species was determined using ten clinical specimens that were positive for Aspergillus galactomannan (using the Bio-Rad PlateliaTM Aspergillus AG kit at an index value cut-off of ³0.5) as well as three specimens spiked with culture filtrates from four different Aspergillus species at different concentrations. Due to the unavailability of clinical specimens with Paracoccidioides brasiliensis, three specimens spiked with culture filtrates from Paracoccidioides brasiliensis at different concentrations were also used to confirm analytic specificity.
Results
We identified seven conference abstracts5-11 and two full-length published articles through August 2012.12-13 Six abstracts and the two full-length articles reported data on serum specimens and five abstracts included data on CSF specimens. (Table 1).
Table 1. Studies on test characteristics of the lateral flow assay in serum and cerebrospinal fluid samples, using three different standards, 2011-2012
In serum specimens, the median sensitivity was 100% (95.6%, 100%) and the median specificity was 99.5% (95.7%, 100%). In CSF specimens, the median sensitivity was 100% (96.2%, 100%) and the median specificity was 97.7% (70.4%, 100%).
The analytic sensitivity of the LFA was demonstrated to be consistently superior to that of the LA or ELISA across all four serotypes by as much as 200-fold (range 19-222-fold). The analytic specificity of the LFA also remained high, with very few instances of cross-reaction. One out of ten specimens that had a positive Aspergillus galactomannan test was LFA positive, at a very low Aspergillus galactomannan index value of 1.04. The range of positive galactomannan index values was 0.68-1.38; specimens with galactomannan index values higher than 1.04 did not demonstrate cross-reactivity, nor did any specimens with high concentrations of Aspergillus species culture filtrates. Antigens from Paracoccidioides brasiliensis exhibited some cross-reactivity at high concentrations. Out of ten specimens with elevated rheumatoid factor levels (range 112 IU/mL to 6479 IU/mL), none were LFA positive. Testing was also performed on five icteric, five hemolyzed, and five lipemic serum specimens, and no reactivity was observed.
One study directly compared the processing times of the LFA, LA, and an EIA. The time it took to process 20 serum specimens was 17 minutes with the LFA, 70 minutes with the LA, and 50 minutes with the EIA.6
Discussion
The available published and publicly available data suggest that the cryptococcal antigen LFA is highly sensitive and specific for the detection of cryptococcal infection in serum and CSF specimens in immunocompromised patients. A direct comparison using different concentrations of glucuronoxylomannan suggests that the LFA has greater analytic sensitivity at lower cryptococcal antigen concentrations than the EIA or LA across all four serotypes. In addition, several studies included an additional referent to reconcile discordant results confirming that the LFA was a true positive when the EIA or LA was negative, thereby further supporting the increased sensitivity of the LFA. For example, when the LA was used as the referent to measure agreement, the EIA was used to reconcile discordant results. The LFA was more often concordant with the “confirmatory” assay than the initial comparison assay. Furthermore, the analytic specificity demonstrated little cross-reactivity.
There were a few limitations to our study. First, the absolute number of published reports was small. Second, our median sensitivities and specificities were calculated using different referents due to the heterogeneity of the study protocols. However, given how tightly clustered the values of the sensitivities and specificities were (with only one outlying specificity measurement), this heterogeneity is not likely significant. Third, we did not include additional unpublished abstracts reporting the sensitivity and specificity of the cryptococcal antigen LFA that we found through communicating with other researchers. We chose to include only peer-reviewed published manuscripts and published scientific conference abstracts in order to ensure the validity of the findings. The published findings were reproducible across different clinical sites and study populations and with different investigators, further enhancing the validity of the results.
The cryptococcal antigen LFA meets the World Health Organization’s ASSURED criteria for being affordable, sensitive, specific, user-friendly, rapid and robust, equipment free, and deliverable to end-users.14 The simplicity of the assay, which can essentially function as a dipstick, makes it user-friendly, requiring little training of laboratory personnel. As with the LA, results may be subject to interpretation. But given the rapid turnaround time and the limited infrastructure needed beyond whole blood collection and serum separation, the accessibility of the LFA is superior to the LA. Furthermore, the data suggest that the test characteristics of the LFA outperform all of the other current standard diagnostic tests for the detection of cryptococcal antigen.
Screening asymptomatic individuals with HIV/AIDS for cryptococcal disease is also more cost-effective with the LFA than with the LA.15 In a region with an approximate 8% prevalence of cryptococcal antigenemia, the LFA would cost $28.37 to detect one case of asymptomatic cryptococcal disease, or less than 15% of the cost of the LA, $190.15 Similarly, when taking into account fluconazole pre-emptive treatment, the use of the LA in a screening and treatment program would cost $266 to prevent one death in a region of similar prevalence, while the LFA would cost $39.73.15
The accuracy of the LFA has yet to be determined in other analytes such as urine, saliva, and whole blood specimens. The testing of such specimens is promising, particularly when used in conjunction with other whole blood or urine point of care tests to measure CD4 T-cell counts and to detect Mycobacterium tuberculosis.16 Given the superior performance, low cost, and comparatively low technological needs of the LFA for serum and CSF specimens, incorporating the cryptococcal antigen LFA in laboratory settings should be prioritized in settings with an increased prevalence of cryptococcosis. Last, consideration should be given to establishing the cryptococcal antigen LFA as the current gold standard for the detection of cryptococcal infection.
The authors, one of whom received a travel grant from a marketer of a lateral flow assay (LFA) for diagnosing cryptococcal infection, summarize the results of studies that suggest the superiority of LFA to latex agglutination (LA) and enzyme immunoassay (EIA) approaches. MLO would be pleased to publish concurring or opposing perspectives.
References
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525-530.
- Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50(8):1101-1111.
- Saha DC, Xess I, Biswas A, Bhowmik DM, Padma MV. Detection of Cryptococcus by conventional, serological and molecular methods. J Medical Microbiol. 2009;58(Pt 8):1098-1105.
- Kozel T, Bauman S. CrAg lateral flow assay for cryptococcosis. Expert Opin Med Diagn. 2012;6(3):245-251.
- Rolfes M, Butler E, von Hohenberg M, et al. Evaluation of a novel point-of- care lateral flow assay to detect cryptococcal antigen in plasma and CSF. In: Conference on Retroviruses and Opportunistic Infections. Seattle;2012.
- Bestrom JE, Jespersen DJ, Rollins L, Binnicker M. An evaluation of four commercial assays for the detection of cryptococcal antigen. In: International Conference on Antimicrobial Agents and Infectious Diseases. San Francisco; 2012.
- Boulware D, Meya D, Longley N, et al. Multicenter evaluation of a novel point- of-care assay for the detection of cryptococcal antigen in HIV-infected persons with and without cryptococcal meningitis. In: ICAAC. San Francisco;2012.
- Clarke S, Gibb R: Evaluation of the IMMY CrAg lateral flow assay for detection of cryptococcal antigen. In: Australian Society for Microbiology. Brisbane; 2012.
- Hansen J, Barker A, Johnson S, et al. Evaluation of a newly developed lateral flow and enzyme-linked immunoassay for the detection of cryptococcal antigen. In: American Society of Microbiology. San Francisco;2012.
- McMullan BJ, Halliday C, Sorrell T, et al. Evaluation of the cryptococcal antigen (CrAg) lateral flow assay in a diagnostic mycology laboratory. Mycoses. 2012; 55:75-76.
- Vijayan T, Bauman S, Chiller S, Klausner J. Test performance of a novel lateral-flow assay to detect cryptococcal disease. In: ID Week. San Diego; 2012.
- Jarvis JN, Percival A, Bauman S, et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV- associated cryptococcal meningitis. Clin Infect Dis.2011;53(10):1019-1023.
- Lindsley MD, Mekha N, Baggett HC et al. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infec dis: 2011.53(4):321-325.
- Peeling RW, Holmes KK, Mabey D, Ronald A. Rapid tests for sexually transmitted infections (STIs): the way forward. Sexually transmitted infect. 2006;82 Suppl 5:v1-6.
- Rajasingham R, Meya D, Rolfes M, Birkenkamp K, Boulware DR. Cost-benefit of integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. In: International AIDS Society Conference. Washington D.C.2012.
- Lawn SD, Wood R. Point-of-care urine antigen screening tests for tuberculosis and cryptococcosis: potential for mortality reduction in antiretroviral treatment programs in Africa. Clinical Infect Dis. 2012;54(5):739-740.