In recent years, a combination of widely publicized
epidemiological studies, clinical trials, and impassioned written and
verbal exchanges among researchers have contributed to the growing
awareness of health professionals and the general public of the many
roles played by vitamin D metabolites. Now, improved vitamin D status
not only is linked to optimized functions of organs considered classical
targets of the vitamin (e.g., bone, intestine, and kidney) but also to
an impressive diversity of tissues.1 As new studies reveal
expanding roles for this vitamin, more clinicians are seeing the
potential benefits of assessing vitamin D status among patients of all
ages on a regular, continuing basis. The means by which vitamin D
adequacy is judged depends upon accurate laboratory determination of
serum 25OH vitamin D concentrations.
The presence of plasma membrane and intracellular
vitamin D receptors (VDRs) has been reported in many cells.1
Selective transcription of more than 200 genes is known to occur in
response to the hormonal form of vitamin D. Gene transcription is
regulated via the hormonal form of vitamin D (1,25(OH)2D)
binding with the intracellular VDR. The physiologic responses triggered
by the targeted genes go far beyond actions related to calcium
homeostasis. In addition to responding to the hormonal form of vitamin D
produced by the kidneys and released into the blood, many
“non-classical, D-influenced” tissues (e.g., colon, breast, prostate)
are capable of producing local 1,25(OH)2D from 25(OHD)D, the
major circulating form of vitamin D. The hormone acting within the
tissues can then exert a remarkable variety of effects, including some
associated with critical actions within tumor microenvironments. Cell
proliferation may be inhibited, differentiation induced, angiogenesis
blocked, and apoptosis promoted — all these effects contributing to
suppression of tumor progression.2,3
All in the “D” family
Vitamin D, a fat-soluble vitamin, is steroid-derived
and is chemically described as a secosteroid because one of its four
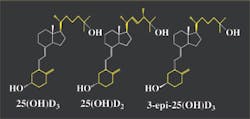
rings is open (see Figure 1).4 The vitamin and its
metabolites exist in two forms.4,5 Those identified as
originating from D2 are derived from yeast and plant material
that has been irradiated with ultraviolet light B or UVB. This form is
only obtained through supplementation and fortification. The D3
forms are found in foods and supplements, and also can be produced by
the body. Structurally, vitamin D2 and vitamin D3
differ in their side chains. The absence of an origin designation (e.g.,
D2 or D3) implies that the material being
discussed is a mixture of both forms, that both forms undergo the
process being described, or that the precise origin of the material to
which reference is made is unknown and could be either D2 or
D3.
The vitamin and its metabolites are transported in
the blood bound to vitamin D-binding protein (DBP), an alpha-2 globulin.5
Although similar in molecular structure (see
Figure 1), D2-derived metabolites have been reported to be
only about one-third to one-half as active as D3 forms of the
vitamin in maintaining serum 25(OH)D serum levels.5 Recent
evidence, however, appears to refute this statement of reduced
effectiveness of D2 derivatives.6
Location, location, location
Vitamin D is one of the few vitamins that can be
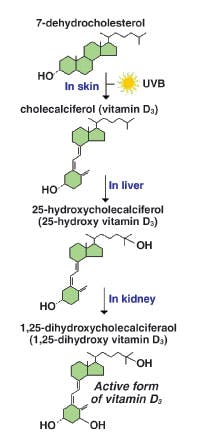
produced by the body. When skin is exposed to UVB radiation (wavelengths
of 290 nm to 320 nm), photolysis of cutaneous 7-dehydrocholesterol
occurs to form vitamin D3.8-10 About 20 minutes of
sunlight exposure during the summer months can produce 20,000 IU of
vitamin D3.9,11 The active form of vitamin D
(1,25(OH)2D) is ultimately produced after two consecutive
hydroxylations, first in the liver and then the kidneys (see Figure 2).
From about October through March, however, UVB
radiation is insufficient to trigger this conversion at locations at or
above 35° N latitude.9 Individuals who spend winter months in
sunny locales and assume that the issue of insufficient cutaneous
synthesis of D3 does not concern them may be surprised to
learn that limited studies have suggested the latitude effect may be a
factor as far south in the United States as 32° N.12
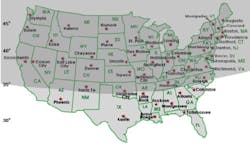
About two-thirds of the U.S. population resides in
regions at or above 35° N latitude (see Figure 3). Many studies have
found a widespread prevalence of vitamin D insufficiency across North
America, especially in the northern regions of the United States and in
Canada.13-16 When discussing this topic, one physician
replied, “It has been difficult to identify a population that is vitamin
D sufficient.”9
Because of diminished natural UVB exposure from fall
through spring months, dietary intake of vitamin D is critical.9
Fatty fish such as salmon, mackerel, and tuna are the best sources, but
fortified foods provide most of the vitamin D in the American diet.17,18
Because of the modest consumption of foods naturally high in vitamin D,
supplementation is usually recommended when UVB exposure is
insufficient.10,18 Regardless of the source, most individuals
do not come close to meeting the current daily reference intake (DRI)
for vitamin D through their diets. Presently, the DRI for adults <50
years of age is 5 µg (200 IU).19 In recent years, there has
been much ardent debate over how much vitamin D is required for
maintenance of adequate levels of 25(OH)D.13 Many authorities
believe that higher intake recommendations for all gender and age
categories are essential.5,9,11 The Institute of Medicine
(IOM) is now beginning the process of reviewing vitamin D requirements,
which many researchers feel are far too low.20
Factors other than geographic latitude, seasonal
fluctuations, and dietary practices impact an individual’s vitamin D
status. Individuals who are older (reduced level of vitamin D precursor
in skin upon aging), have darker skin (higher melanin content), and
whose clothing or sunscreen covers most of their skin, have a decreased
ability to convert 7-dehydrocholesterol to 25(OH)D3, even
when adequate sunlight is obtained.9,21 One study of elderly
adults living in south Florida found approximately 40% of their sample
population was vitamin D deficient during the winter months.22
National Health and Nutrition Examination surveys
(NHANES) have been conducted since 1971 with the goals of examining the
health and nutritional status of non-institutionalized Americans. The
NHANES III was conducted between 1988 and 1994 by the National Center
for Health Statistics of the Centers for Disease Control and Prevention
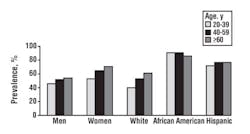
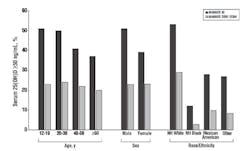
(CDC).21 The report of the survey exposed gender, racial, and
ethnic differences in 25(OH)D concentrations in various population
sectors; women had lower concentrations than men, non-Hispanic whites
had higher concentrations than Mexican-Americans, who in turn had higher
concentrations than non-Hispanic blacks, and leaner and more active
women had higher concentrations than heavier and less-active women (see
Figure 4).21 Overall, about two-thirds of the U.S. population
had serum levels indicating insufficiency.23
Recently published data based from NHANES 2001-2004
indicates three of every four Americans have insufficient levels of
25(OH)D; 77% had insufficient levels of vitamin D.24 Overall,
the prevalence of 25(OH)D levels of 30 ng/mL or more decreased by
approximately half from 45% in NHANES III to 23% in NHANES 2001-2004
(see Figure 5). Ninety-seven percent of non-Hispanic blacks and 90% of
Mexican-Americans meet the criteria for being vitamin D insufficient.
The suggestion has been made that minorities are at a markedly increased
risk of health problems due — at least, in part — to poor vitamin D
status.24
Clinical applications
Approximately 35 tissues in humans have been
identified as possessing VDRs.1 Cells and tissues that are
known to contain VDR receptors include the brain, heart, skin, gonads,
prostate, breast, adipose tissue, adrenals, bone and bone marrow,
cartilage, gastrointestinal tract and associated organs, immune cells
(including activated lymphocytes, B and T cells), cardiac and smooth
muscle cells, β-cells
of the pancreas, thyroid gland, and parathyroid gland.1 VDR
binds several forms of D, but its affinity for 1,25(OH)2D3
is roughly 1,000 times that for 25OHD3.6 Table 1
identifies some conditions in which vitamin D insufficiency appears to
play a role. As appreciation of the critical functions of vitamin D
metabolites grows, practitioners in medical specialties such as
geriatrics, orthopedics, physical medicine and rehabilitation, oncology,
rheumatology, and family practice are increasingly likely to see major
benefits in ordering testing of patients to assess their vitamin D
status.5,25
Poor vitamin D status may increase risk of:
- nervous system disease;
- multiple sclerosis;
- schizophrenia;
- depression;
- diabetes type 1 and 2;
- obesity;
- hypertension;
- muscle weakness (even in adolescents);
- frailty syndrome in older adults;
- heart failure;
- cardiovascular disease;
- cancers (i.e., colorectal, breast, prostate, non-melanoma skin);
- psoriasis;
- infectious diseases (i.e., tuberculosis, leprosy, seasonal epidemic influenza virus type A);
- asthma and allergy severity in childhood;
- polycystic ovary disease, menstrual problems and fertility;
- Crohn’s and other inflammatory bowel diseases;
- bone disease (i.e., rickets, osteomalacia, osteoporosis); and
- periodontal disease.
Measuring vitamin D
How much constitutes enough? Globally, approximately 1 billion individuals are deficient in or have
insufficient circulating levels of 25(OH)D.18 The actual
magnitude of vitamin D inadequacy is undoubtedly greater. As reflected
in measurements of circulating levels of 25(OH)D, insufficiency of
vitamin D has reached epidemic proportions in North America. Signs and
symptoms linked to poor vitamin D status are often non-specific until
deficiency is severe and bone integrity is compromised.26
Patient complaints of chronic muscle and bone pain, depression, fatigue,
cognitive difficulties, loss of muscle strength and mass, poor balance,
and falls are attributable to multiple causes (or may simply be
disregarded, especially if the patient is elderly).26 Only
accurate laboratory assessment of serum 25(OH)D can focus attention on
the true cause of the patient’s difficulties and guide the clinician in
taking corrective action.
Serum concentrations of 25(OH)D correlate with
substrate levels of vitamin derived both from dietary intake and its
cutaneous synthesis.8 A total of 100 IU (2.5 µg) of vitamin D
raises the blood level of 25(OH)D by 1 ng/mL.27 The
biologically active form of vitamin D, 1,25(OH)2D, is not
routinely measured by the laboratory.8 Its level is tightly
regulated by physiologic factors, and its half-life is only a few hours;
conversely, serum 25(OH)D’s half-life is several weeks and is,
therefore, a suitable reflection of the patient’s actual vitamin D
status.
Establishment of what serum level of 25(OH)D is
sufficient (or universally best) remains unsettled.9,10,13,18
Since the influence of vitamin D in the body is widespread, it becomes
problematic to pinpoint the value that is adequate or, better yet,
optimal year-round for all vitamin D-related physiological processes.1
For instance, the value that is most favorable for calcium homeostasis
and bone health may be different than that concentration that is deemed
best for immune or cardiovascular function. Application of different
criteria increases the difficulty of achieving consensus as to what
constitutes the “ideal” serum level of 25(OH)D. Table 2 shows the
typical values used to indicate various levels of vitamin D sufficiency.
Testing frequency. Currently, no formal
recommendations exist as to when and how frequently vitamin D testing
should occur, so the testing is left to the discretion of clinicians. It
is strongly recommended that all patients who are at risk of vitamin D
deficiency be tested.10 Seasonal variations in the analyte
concentration must be taken into account, so one test is inadequate to
establish sufficiency of 25(OH)D. Testing should be conducted at least
twice a year; measuring vitamin D levels during the fall through spring
seasons would allow clinicians to assess the impact of “winter drop-off”
on the adequacy of 25(OH)D circulating levels.
Automation, high sample throughput, and shortened
turnaround time have contributed to a dramatic increase testing
requests.28 Predictions called for several million tests
assessing vitamin D status to be conducted in the United States by the
end of 2008. In fact, 25(OH)D testing has surged by as much as 80% to
90%.29 A test that was once a rarity and thought to be
suitable primarily for a research setting is now seen as a valuable
diagnostic tool appropriate for the clinical laboratory. Despite this,
there are serious issues associated with testing, such as the high
variability in analyte measurement due to intermethod variability,
laboratory-to-laboratory variation, and lack of standardization against
reference materials.25
Comparing assay technologies. Vitamin D
assays originally were a time-consuming procedure, measuring DPB as the
primary binding agent and a tritium-labeled tracer (3H-25(OH)D3).4
Tests currently in use are much more appropriate to a high-throughput
laboratory and include various forms of immunoassay, high-performance
liquid chromatography (HPLC) and tandem mass spectrometry (MS/MS).8,30
The need to address the variability that exists among different assay
methods to assess vitamin D status is a priority.31
- Immunoassays. Immunoassays are
extensively used and have been in previous large, ongoing studies,
such as NHANES (CDC), the Harvard Nurses’ Study, the Harvard Health
Professions Study, and the Framingham Study. LabCorp (Burlington,
NC), the second largest reference laboratory-testing company in the
United States, employs this method to assess 25(OH)D concentrations. - Radioimmunoassay (RIA). Developed in
1985, this method is produced and marketed by DiaSorin (Stillwater,
MN). RIA accurately measures total 25(OH)D but is unable to separate
D2 and D3. Despite this, RIA is frequently
used because of its high correlation with HPLC and minimal
procedural steps. Despite minimal processing, there can be a high
cost per test.25 - Chemiluminescent immunoassay (CLIA). The
LIAISON 25(OH)D assay has largely replaced the RIA and is also
produced and marketed by DiaSorin. CLIA is fully automated and its
results are highly correlated with RIA. Roche Diagnostics
(Indianapolis, IN) has recently introduced the Elecsys 25(OH)D3
assay for use with the company’s immunoassay analyzers.32
Serum 25(OH)D can also be measured using the Nichols ADVANTAGE
25(OH)D chemiluminescent assay (Nichols Institute, San Clemente,
CA).
| Level of sufficiency | Value |
| Severe deficiency | |
| Vitamin D deficiency | |
| Vitamin D insufficiency | 21 ng/mL to 29 ng/mL (52 nmol/L to 72 nmol/L) |
| Adequate to derive health benefits | 30 ng/mL to 100 ng/mL (75 nmol/L to 250 nmol/L) |
| Table 2. Levels of vitamin D sufficiency. 5,9,17,18 |
|
| Clinical laboratories reporting serum measurements of 25(OH)D may use conventional units (ng/mL) or international system (SI) units (nmol/L). The conversion factor to SI units is: 1 ng/mL = 2.496 nmol/L.13 | |
If previous reports of the D2 derivatives
being substantially less effective than the D3 derivative in
maintaining vitamin D status are correct (though as noted earlier recent
evidence contradicts that assertion5), then a diagnostic
dilemma may be created when an assay procedure does not distinguish
between D2 and D3 levels. In theory, a patient
could have a total 25(OH)D value fall within the acceptable range, but
the bioactivity of the total 25(OH)D could actually be considerably less
than ideal. A situation similar to a false-negative finding would exist,
in which case, the patient would not then be treated for hypovitaminosis
D when, in fact, clinically, they would be at less than a desirable
level of the vitamin.
- LC-MS/MS. Liquid chromatography tandem
mass spectrometry (LC-MS/MS) is considered the “method of choice” by
many, because this method is able to separate and individually
quantify 25(OH)D2 and 25(OH)D3. The technique,
however, is not without shortcomings.14,29,33 There have
been numerous reports of discrepancies between the results of
LC-MS/MS and immunoassays, with LC-MS/MS reporting serum values up
to 40% higher than those reported using RIA.33 Survey
data have indicated that labs performing 25(OH)D immunoassays report
results ranging from 41 ng/mL to 96 ng/mL for a survey sample with a
value of 75 ng/mL determined by LC-MS/MS.29 Because of
this, the method of testing is crucial to note when interpreting
laboratory values.33
Additional challenges with using this method properly
include the need for each site to develop its own in-house standards
(calibrators), since there is as yet no certified reference material to
test method accuracy and a high-degree of expertise of laboratory
personnel required to conduct the complex procedure.14 In
addition, the lack of standardization among laboratories is a crucial
concern.14 Not all laboratories have the same standard
operating procedures, and LC-MS/MS interlaboratory value can vary by
20%.29 Aliquots of one sample sent to seven different
laboratories using LC-MS/MS to measure vitamin D reportedly generated
seven different results in one study.33 Mayo Medical
Laboratories (Rochester, MN) employs this methodology, as does Quest
Diagnostics (Madison, NJ), the largest reference laboratory-testing
company in the United States.
Problems with testing
Not all test procedures are equivalent. This may
explain the recent brouhaha over vitamin D testing conducted by Quest
Diagnostics (Madison, NJ).33 Quest produced its own assay for
LC-MS/MS in 2006. Shortly thereafter, many physicians noticed the values
were inconsistent with those obtained previously for specific patients.
In late 2008, the corporation contacted thousands of physicians,
indicating values reported on patients might not have been accurate and
offered retesting at no charge. Word spread rapidly, and the details of
the unfolding saga were revealed in The Dark Report of December
22, 2008. The Quest retest offer became public knowledge when an article
appeared in The New York Times on January 7, 2009, describing the
astonishing situation. Serious questions regarding overall lab testing
accuracy and oversight have since been raised.11,33
While any well-run laboratory can experience an error
in testing at some point, this error is usually quickly recognized and
corrected.33 In the case of Quest Diagnostics, the error went
unnoticed by laboratory personnel for 18 months to 24 months. It has
been suggested that the pressure on both the company’s personnel and its
LC-MS/MS platform due to the rapid increase in 25(OH)D testing that
occurred at Quest could explain why the error went unremarked for so
long. Problem areas identified were reagent preparation and
inconsistencies in operating procedures. In a corporate statement, Quest
claims to have corrected the problem by the end of 2008.
Despite the nature of this incident, it has value in
serving as a dramatic reminder to all laboratories of the importance of
strict adherence to quality-control and quality-assurance practices. It
highlights the obligation of the laboratory to promptly identify and
rectify the problem and to swiftly communicate with all clients the
possibility of erroneous results having been reported. Credibility once
questioned is not easily regained.
Improving testing
Calibration of D2 and D3 assays
has been a continuing problem because there is no certified reference
material available to test method accuracy.34 The National
Institute of Standards and Technology is currently creating a
serum-based Standard Reference Material (SRM) to be used as a control
material by labs. This could reduce the interlaboratory variability of
measurements and enhance the accuracy of nutritional status data in the
NHANES survey.35 The standard, identified as SRM 729, was
expected to be released by the end of 2008 but is not yet available.
Some researchers suggest that using serum vitamin D levels from a
healthy population would be the best reference material for assessing
adequacy.1 The SRM will possess variability similar to that
seen in a healthy vitamin D sufficient population, by consisting of four
pools with varying levels of 25(OH)D2 and 25(OH)D3.31
One pool will also contain 3-epi-25(OH)D3, a form of vitamin
D that has been found to be present in infants (see Figure 1).
The UK-based Vitamin D Quality Assessment Scheme
(DEQAS) has been monitoring the performance of 25(OH)D assays since 1989
and consists of approximately 400 participating laboratories worldwide.4,36
The overall aim of DEQAS is to ensure the analytical reliability of
25(OH)D and 1,25(OH)2D assays.37 DEQAS provides an
opportunity to assess the accuracy and specificity of 25(OH)D methods as
well as monitoring the analytical performance of a large number of their
users.4
An improvement in vitamin D testing has recently been
developed by ZRT Laboratory (Beaverton, OR) that will facilitate
large-scale testing of vitamin D status.38 Sample collection
is via finger-stick rather than venipuncture, and the analysis of the
dried blood spot is by LC-MS/MS. The test is reported to be convenient,
cost effective, and reliable. The test was found to highly correlate
with traditional LC-MS/MS testing and was also able to separate D2
from D3.
The future
The existence of an epidemic of vitamin D
insufficiency in the United States is confirmed by measurements of
circulating levels of 25(OH)D showing almost 75% of adults and
adolescents possess serum 25(OH)D levels less than 30 ng/mL.24
On the brighter side, a newly published article indicated that daily
intakes of 3.5 µg of dietary vitamin D and 20 µg of vitamin D3
supplements during winter was enough to achieve adequate serum 25(OH)D
concentrations in 80% of premenopausal women in Maine by winter’s end.39
As more clinicians begin to see the medical
importance of knowing a patient’s vitamin D status — information readily
acquired by ordering serum 25(OH)D testing for their patients —
understanding the basics of vitamin D formation and its actions, and
being aware of the challenges associated with meaningful laboratory
assessment of this analyte are crucial.
Circulating 25(OH)D measurement offers an important
clinical tool in the diagnosis, management, and prevention of a variety
of disease states. Laboratory professionals and clinicians also need to
be conscious of the technical challenges associated with both measuring
and interpreting 25(OH)D levels. For maximum diagnostic accuracy, assay
standardization must be achieved along with identification of a
consistent reference range for circulating 25(OH)D levels.
Lynn Stiff,
a Nutrition and Dietetics graduate student and dietetic intern at
Northern Illinois University (NIU), at DeKalb, IL, will receive her MS
degree in December upon completion of her dietetic internship. She
received her BS in Human Biology (concentration: Nutritional Sciences)
from the University of Wisconsin-Green Bay.
Sharon M. Miller, PhC, MT(ASCP), CLS(NCA),
is professor emerita at NIU as well as an MLO editorial advisory
board member. She teaches NIU graduate courses in nutritional
biochemistry (specifically micronutrients). Her longstanding interest in
vitamin D has inspired Miller to write numerous articles and chapters in
clinical laboratory sciences textbooks on vitamins and minerals. She has
also served as chair of AACC’s Nutrition Division.
References
- Norman AW. From vitamin D to hormone D:
Fundamentals of the vitamin D endocrine system essential for good
health. Am J Clin Nutr. 2006;88;491S-499S. - Deeb KK, Trump DL, Johnson CS. Vitamin D
signaling pathways in cancer: Potential for anticancer therapeutics.
Nature Rev Cancer. 2007;7:684-700. - Bikle D. Nonclassic actions of vitamin D. J
Clin Endocrinol Metab. 2009;1:26-34. Epub. October 14, 2008. - Hollis BW. Measuring 25-hydroxyvitamin D in a
clinical environment: Challenges and needs. Am J Clin Nutr.
2008;88(supp):507S-510S. - Shinchuk L, Holick MF. Vitamin D and
rehabilitation: Improving functional outcomes. Nutr Clin Pract.
2007;22:297-304. - Bowen R. Vitamin D (Calcitriol).
Pathophysiology of the Endocrine System. Colorado State
University. October 2007. Available at:
http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/otherendo/vitamind.html.
Accessed January 29, 2009. - Hollick MF, Biancuzzo RM, Chen TC, Klein EK, et
al. Vitamin D2 is as effective as vitamin D3
in maintaining circulating concentrations of 25-hydroxyvitamin D.
J Clin Endocrinol Metab. 2008;93:667-681. - Zerwekh J. Blood biomarkers of vitamin D status.
Am J Clin Nutr. 2008;87:1087S-1091S. - Cannell JJ, Hollis BW. Use of vitamin D in
clinical practice. Alt Med Rev. 2008;13:6-20. - Moyad MA. Vitamin D: A rapid review. Urologic
Nurs. 2008;28:343-384. - Vitamin D Council. Understanding vitamin D
cholecalciferol. Available at:
http://www.vitamindcouncil.org/.
Accessed January 18, 2009. - Basile LA, Taylor SN, Wagner CL, Quinones L,
Hollis BW. Neonatal vitamin D status at birth at latitude 32°72′:
Evidence of deficiency. J Perinatol. 2007;27(9):568-571. - Department of Health and Human Services: Centers
for Disease Control and Prevention. National report on biochemical
indicators of diet and nutrition in the U.S. Population 1999-2002.
July 2008. Available at:
http://www.cdc.gov/nutritionreport/pdf/nutrition_report.pdf.
Accessed January 18, 2009. - Hanley DA, Davison KS. Vitamin D insufficiency in
North America. J Nutr. 2005;135:332-333. - Schwalfenberg G. Not enough vitamin D. Can Fam
Physician. 2007;53:841-854. - Higdon J. Preventing osteoporosis through diet
and lifestyle. Linus Pauling Institute Spring/Summer 2005
Research Report. May 2005. Available at:
http://lpi.oregonstate.edu/ss05/osteoporosis.html. Accessed January
25, 2008. - Dietary Supplement Fact Sheet: Vitamin D.
National Institute of Health: Office of Dietary Supplements.
2008. Available at:
http://ods.od.nih.gov/factsheets/vitamind.asp.
Accessed January 26, 2009. - Holick MF. Medical progress: Vitamin D
deficiency. N Eng J Med. 2007;357:266-281. - Standing Committee on the Scientific Evaluation
of Dietary Reference Intakes. DRI Dietary Reference intakes for
calcium, magnesium, vitamin D, and fluoride. Institute of
Medicine Food and Nutrition Board. 1997. National Academy Press;
Washington D.C. Available at:
http://books.nap.edu/openbook.php?record_id=5776&page=250.
Accessed January 18, 2009. - Yetley EA, Brule D, Cheney MC, Davis CD, et al.
Dietary Reference Intakes for vitamin D: justification for a review
of the 1997 values. Am J Clin Nutr. 2009;89:1-9. - Yetley EA. Assessing vitamin D status of the US
population. Am J Clin Nut.. 2008;88:558S-564S. - Levis S, Gomez A, Jimenez C, Veras L, et al.
Vitamin D deficiency and seasonal variation in an adult south
Florida population. J Clin Endocrinol Metab.
2005;90(3):1557-1562. - Martins D, Wolf M, Pan D, Zadshir A, et al.
Prevalence of cardiovascular risk factors and the serum levels of
25-hydroxyvitamin D in the United States: Data from the third
National Health and Nutrition Examination Survey. Arch Intern Med.
2007;167:1159-1165. - Ginde AA, Liu MC, Camargo Jr. CAC. Demographic
differences and trends of vitamin D insufficiency in the US
population, 1988-2004. Arch Intern Med. 2009;169(6):626-632. - Chandler DW, Hollis BW. Assessment of vitamin D
status: Methods, levels, and medical consequences. American
Society for Clinical Pathology: 2008 Annual Meeting, Baltimore, MD.
October 16, 2008. Available at:
http://www.ascp.org/LongDescriptions/ASCPCourseMaterials/AnnualMeetingMaterials.aspx.
Accessed February 1, 2009. - Shardell M, Hicks GE, Miller RR, Kritchevsky S,
et al. Association of low vitamin D levels with the frailty syndrome
in men and women. J Gerontol A Biol Sci Med Sci.
2009;64A(1):69-75. - Holick M. Vitamin D and sunlight: strategies for
cancer prevention and other health benefits. Clin J Am Soc
Nephrol. 2008;3:1548-1554. - Hollis BW, Horst RL. The assessment of
circulating 25(OH)D and 1,25(OH)2D: Where we are and
where we are going. J Steroid Biochem Mol Biol.
2007;103:473-476. - Singh RJ. Are clinical laboratories prepared for
accurate testing of 25-hydroxy vitamin D? Clin Chem.
2008;54:221-223. - Hollis BW. The determination of circulating
25-hydroxyvitamin D: No easy task. J Clin Endocrinol Metabolism.
2004;89(7):3149-3151. - Phinney KW. Development of a standard reference
material for vitamin D in serum. Am J Clin Nutr.
2008;88:511S-512S. - Leino A, Turpeinen, Koskinen P. Automated
measurement of 25OH Vitamin D3 on the Roche Modular E170
Analyzer. Clin Chem. 2008;54:2059-2062. - Dark RL. Inaccurate vitamin D results result in
patient retest/recall program! The Dark Report.
2008;15(17):1-19. - Yates AM, Bowron A, Calton L, Heynes J , et al.
Interlaboratory variation in 25-Hydroxyvitamin D2 and
25-Hydroxyvitamin D3 is significantly improved if common
calibration material is used. Clin Chem. 2008;54:2082-2084. - Phinney KW, Sanders LC, Sharpless KE, Wise SA.
NIST develops serum-based standard reference materials to assess
nutritional status. Chemical Science and Technology Laboratory,
National Institute of Standards and Technology: Food and Nutrition.
March 2007. Available at:
http://www.cstl.nist.gov/projects/fy06/food0683904.pdf.
Accessed April 1, 2009. - Carter GD, et al. How accurate are assays for
25-hydroxyvitamin D? Data from the International Vitamin D External
Quality Assessment Scheme. Clin Chem. 2004;50(11):2195-2197. - DEQAS Advisory Panel. About DEQAS. DEQAS:
Vitamin D External Quality Assessment Scheme. Available at:
http://www.deqas.org. Accessed April 2, 2009. - Newman MS, Brandon TR, Groves MN, Gregory WL,
Kapur S, Zava DT. A Liquid Chromatography/Tandem Mass Spectrometry
Method for Determination of 25-Hydroxy Vitamin D2 and
25-Hydroxy Vitamin D3 in Dried Blood Spots: A Potential
Adjunct to Diabetes and Cardiometabolic Risk Screening. J
Diabetes Sci Tech. 2009;3:156-162. - Nelson ML, Blum JM, Hollis BW, Rosen C, Sullivan SS. Supplements of
20 µg/d cholecalciferol optimized serum 25-hydroxyvitamin D
concentrations in 80% of premenopausal women in winter. J Nutr.
2009;139:540-546.