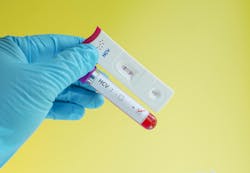
The World Health Organization (WHO) has prequalified the first hepatitis C virus (HCV) self-test which can provide a critical support in expanding access to testing and diagnosis, accelerating global efforts to eliminate hepatitis C.
The product, called OraQuick HCV self-test, manufactured by OraSure Technologies, is an extension of the pre-qualified, OraQuick HCV Rapid Antibody Test which was initially prequalified by WHO in 2017 for professional use. The self-test version, specifically designed for use by lay users, provides individuals with a single kit containing the components that are needed to perform the self-test.
WHO’s prequalification (PQ) program for in vitro diagnostics (IVDs) evaluates a range of tests, including those used for the detection of antibodies to HCV. The program assesses IVDs against quality, safety and performance standards.
WHO will continue to assess additional HCV self-tests, support evidence-based implementation, and work with communities to expand available options to all countries.