Twelve steps to CD4 testing—Part III: Management of opportunistic infections
The 2016 and 2017 World Health Organization (WHO) guidelines provide guidance on the diagnosis of human immunodeficiency virus (HIV) infection, the care of people living with HIV, and the use of antiretroviral (ARV) drugs for treating and preventing HIV infection.
While these guidelines recommend lifelong antiretroviral therapy (ART) regardless of CD4 cell count (“treat all policy”) and analysis of viral load (VL) as the preferred monitoring approach, they also provide clear guidance on the indispensable role of CD4 in assessing baseline risk of disease progression—particularly for individuals presenting with advanced disease—decisions regarding starting and stopping prophylaxis for opportunistic infections (OIs), and prioritization decisions regarding ART initiation in settings where universal treatment is not possible. CD4 cell count measurement may also be important for people who are failing ART.
As a recap, the following is a continuation from last month’s “HIV treatment.” We now continue with “Management of opportunistic infections.”
7. PROPHYLAXIS INTERVENTIONS
Prophylaxis interventions for people with advanced HIV disease
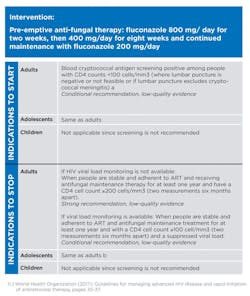
Co-trimoxazole (CTX) prophylaxis is recommended for adults (including pregnant women) with severe or advanced HIV clinical disease (WHO stage 3 or 4) and/or with a CD4 count <350 cells/µl.10,11Co-trimoxazole prophylaxis is recommended for infants, children and adolescents with HIV, irrespective of clinical and immune conditions. Priority should be given to all children younger than 5 years old regardless of CD4 cell count or clinical stage, and children with severe or advanced HIV clinical disease (WHO clinical stage 3 or 4) and/or those with a CD4 count <350 cells/µl.10,11Pre-emptive antifungal therapy: In adults and adolescents, if blood cryptococcal antigen screening positive among people with CD4 counts <100 cells/µl (where lumbar puncture is negative or not feasible or if lumbar puncture excludes cryptococcal meningitis).128. MANAGEMENT OF OPPORTUNISTIC INFECTIONS
Identification of opportunistic infections: Tuberculosis (TB)
Except as specifically described below for people with HIV infection with low CD4 counts or who are seriously ill, urine lateral flow (LF)-LAM should not be used for the diagnosis of TB.12
LF-LAM may be used to assist in the diagnosis of active TB in adult inpatients living with HIV, with signs and symptoms of TB (pulmonary and/or extrapulmonary), who have a CD4 count less than or equal to 100 cells/µl, or people living with HIV who are seriously ill, regardless of CD4 cell count or with unknown CD4 cell count.12 LF-LAM should not be used as a screening test for active TB.12
Identification of opportunistic infections: Cryptococcus spec.
The use of routine serum or plasma Cryptococcus antigen (CrAg) screening in ART-naive adults, followed by pre-emptive antifungal therapy if CrAg positive to reduce the development of cryptococcal disease, may be considered prior to ART initiation in:13
a. patients with a CD4 count less than 100 cells/µl; and
b. where this population also has a high prevalence (>3 percent) of cryptococcal antigenaemia.
Identification of opportunistic infections: Skin and oral conditions
HIV infection increases the prevalence and severity of skin and oral diseases, especially when the person’s CD4 count declines below 200 cells/µl. As a result, skin and oral conditions affect up to 90 percent of adults and children with HIV in resource-limited settings.14
Certain systemic diseases, such as Kaposi sarcoma, may initially be noted on the skin and may require urgent ART to reduce mortality. Others, while not always a major cause of mortality, can be a source of severe morbidity through, for example, itching that provokes scratching, secondary infections, disfigurement, sleep disturbance, and psychological stress. In the case of candidiasis, it can cause pain on swallowing, limiting a person’s ability to take ARV drugs.14
9. VACCINATION SCHEMES
Vaccination Scheme: Measles
Vaccines usually have better safety and efficacy among people with HIV who are receiving ART and those without significant immunosuppression, notably when the CD4 count is above 200 cells/µl.14 People with more severe immunosuppression may be at higher risk of complications from some live attenuated vaccines.14
Vaccination should be routinely administered to potentially susceptible, asymptomatic children and adults living with HIV and should be considered for those with symptomatic HIV infection if they are not severely immunosuppressed according to WHO definitions (CD4 cell counts < 50 cells/µl).15
Vaccination Scheme: Yellow Fever
Yellow fever vaccine may be offered to asymptomatic people living with HIV with CD4 cell counts >200 cells/µl; it is therefore contra-indicated in people with advanced HIV disease until they achieve a CD4 cell count >200 cells/µl. Although the data on the safety and immunogenicity of yellow fever vaccine when used among children living with HIV are limited, yellow fever vaccine may be administered to all clinically well children. HIV testing is not a prerequisite for vaccination.15
This is the last MLO Special Feature outlining Beckman Coulter Life Sciences’ technical overview of “12 Steps to CD4 Testing.” For more information, visit https://www.beckman.com/about-us/cares/initiative.
REFERENCES
10. World Health Organization (2016): Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; recommendations for a public health approach – 2nd ed., page 193.
11. World Health Organization (2017): Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy, pages 35-37.
12. World Health Organization (2016): Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; recommendations for a public health approach – 2nd ed., page 197.
13. World Health Organization (2016): Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; recommendations for a public health approach – 2nd ed., page 205.
14. World Health Organization (2016): Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; recommendations for a public health approach – 2nd ed., page 214/215.
15. World Health Organization (2017): Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy, page 16-17.