The importance of external quality assessment in HIV enzyme immunoassay testing
Quality Control (QC) is vital for the clinical laboratory in order to ensure the accuracy and precision of patient test results. Without a robust QC strategy, test system errors can go undetected, potentially resulting in misdiagnosis and inappropriate/delayed patient treatment.
QC can be divided into two main components; Internal Quality Control (IQC) and External Quality Assessment (EQA).
What are IQC and EQA?
IQC is used in the daily monitoring of test system precision and reproducibility. IQC essentially compares the internal laboratory’s performance over time, highlighting any deviations from “normal” performance. While IQC is a good method of testing performance, it is not always robust enough to detect calibration errors or issues associated with “wide” acceptable limits.
In an EQA program (also known as Proficiency Testing, or PT), the EQA provider will deliver “blind” samples to all EQA participants. The use of such samples ensures that EQA can be used to measure a laboratory’s bias and accuracy. These samples are analyzed by the lab and results are returned to the EQA provider for analysis. The data are examined, means and SDs are calculated, and any outliers are highlighted. Reports are then generated and delivered to each participant, giving a summary of performance in comparison to the rest of the participant group.
Why carry out EQA testing?
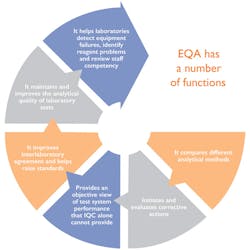
The World Health Organization (WHO) recommends that a robust QA program be implemented in any lab which carries out HIV testing, and Internal and External quality control should be carried out on an ongoing basis.1 In addition, participating in a rigorous and robust EQA scheme is a requirement of ISO 15189. All labs seeking ISO 15189 accreditation need to be part of an EQA scheme which appropriately challenges their test system. Figure 1 shows some of the functions of an EQA program.
The cost of poor quality EQA
The most common method of HIV detection is enzyme immunoassay (EIA) antibody testing.2 EIA tests are highly sensitive and can be optimized for automation, facilitating high-volume testing. However, with higher volumes of testing comes a higher probability of error. With EIA assays, the most common error encountered is false positive results.2
False positive results have a number of implications. Any screen-positive results must be verified using a confirmatory test, usually a Western Blot assay. The Western Blot is much more labor-intensive and expensive than EIA tests, and it is not efficient enough for high-volume testing.2 Therefore, if a laboratory is producing a higher than average number of false positive results due to poor quality assurance practices, it will ultimately spend significantly more time and money on confirmatory tests.
As HIV is classified as a pandemic and a chronic illness, it is of the utmost importance that testing be accurate and consistent. If errors arise, false positive and false negative results can have serious implications for both the patient and the laboratory itself. If incorrect results are reported, patients could potentially file lawsuits, the lab’s reputation will be tarnished, and regulatory bodies could strip the lab of its accreditation status.
How EQA can improve HIV testing accuracy
The main use of EQA is to verify the accuracy of laboratory testing. Accuracy refers to the closeness of the obtained result to the “true” value. As EQA is tested using blind samples, operator bias is eliminated and the test system can be appropriately challenged. EQA can also be used to identify pre-analytical, analytical, and post-analytical errors:
- Pre-analytical involves issues with sample/reagent preparation, handling, etc.
- Analytical can include instrument and calibration issues.
- Post-analytical may involve issues with the interpretation of analytical results and general human error.
EQA can also be used as a means of detecting deficiencies with IQC. For example, some IQC providers supply assayed IQC material with very wide acceptable limits, with 2SD often +/- 20 percent from target value. For this reason, labs may believe their performance is good, but when scrutinized by EQA with narrow acceptable limits, performance will be identified as substandard.
What to look for in a good EQA scheme
Laboratories can join Local, National or International EQA schemes. An International EQA scheme provides the best return on investment due to the higher peer group size and more diverse demographic of participants. Some key factors which typify a good quality EQA scheme include the following
- Accreditation to ISO 17043: Being accredited to this standard ensures the EQA scheme is fit for purpose. ISO 17043 is an all-encompassing standard that accounts for
° design and implementation of the EQA scheme
° sample quality, manufacture, storage, and transport
° statistical practices used to determine participant performance
° participant confidentiality. - Frequent distribution: Regular EQA analysis allows for rapid identification of errors, and any necessary corrective actions can be taken as soon as possible.
- Large number of participants: This ensures an extensive database of results for a range of analytical methods and instruments. In addition, larger participant groups increase the statistical validity of results.
- Clinically relevant ranges: Analytes should be present at both normal and abnormal ranges, as to ensure medical decision limits are adequately tested.
- Comprehensive reports: Much of the value of participating in an EQA scheme lies in the report generated by the provider. Reports should be comprehensive, providing a range of statistical metrics and charts.
Early warning for the lab
An EQA scheme operates like an early warning system; it can alert the lab and document the need for, stimulate, and monitor improvement. However, the improvements themselves must be implemented and maintained by the lab. Investment in a good EQA scheme can save labs a substantial amount of time and money, so it is worthwhile to invest wisely.
REFERENCES
- Laboratory Methods For Diagnosis of HIV Infection In Infants And Children. Geneva: World Health Organization. 2010;5.
- Fearon M. The laboratory diagnosis of HIV infections. The Canadian Journal of
Infectious Diseases & Medical Microbiology. 2005;16(1):26-30.
Edward Hill, BSc, serves as a QC Product Specialist for Randox Laboratories, provider of Laboratory Quality Controls, Peer Group Reporting Software, and other diagnostic solutions for clinical, food/wine testing, veterinary, forensic, and molecular laboratories.