2025 MLO State of the Industry Survey: Labs respond to evolving needs in Alzheimer’s/dementia and respiratory testing amid global supply chain uncertainties
The 2025 Medical Laboratory Observer (MLO) State of the Industry (SOI) survey on Disease Management focused on Alzheimer’s/dementia and respiratory testing. With regards to the former, MLO queried medical laboratory professionals about clinical reasons for Alzheimer’s/dementia test ordering, specific biomarkers tested, methodologies used, and number of tests performed over the past year.
As for the latter, participating medical lab professionals responded to questions on respiratory testing volumes, viruses for which they have seen the most positive results, and types of tests their labs are using to diagnose respiratory viruses.
Given the uncertainties related to the global supply chain, MLO also asked medical lab professionals whether geopolitical factors were affecting their labs’ purchasing/inventory management strategies.
Among the survey respondents, nearly half (48%) hold laboratory director, manager, administrator, or supervisor positions and the majority (62%) are employed by health system or hospital labs. The number of employees who work in their labs spanned the spectrum, with 29% reporting 1-10 employees, 23% more than 100, and the others falling in ranges in between.
Alongside the quantitative results, we include commentary from medical lab professionals and vendors in the Alzheimer’s/dementia and respiratory testing spaces.
Alzheimer’s/dementia testing trends
When asked how many tests their labs had performed for Alzheimer’s/dementia in the past 12 months, nearly half of lab professionals (48%) selected the low end of the volume spectrum (0-100 tests) while 9% reported performing high test volumes (500+ tests).
Additionally, 4% of survey respondents performed 101-250 tests and 2% 251-500 tests. More than one-third of lab professionals (36%) indicated they were unsure how many Alzheimer’s/dementia tests their labs had performed in the past year.
Test modalities
Turning to Alzheimer’s/dementia testing methodologies, the most used among lab professionals surveyed was complete blood count (44%), followed closely by urinalysis (40%), blood glucose (40%), and analysis of thyroid and thyroid-stimulating hormone levels (36%). One-quarter of respondents use drug and alcohol testing (25%), 24% cerebrospinal fluid analysis, and 20% toxicology testing.
More than half of respondents (51%) reported using other testing methodologies. When asked specifically what they used, lab professionals cited heavy metals testing, comprehensive metabolic panel (CMP) blood tests, APO lipoprotein panel, and tests to rule out prion diseases and other conditions.
Several lab professionals commented on how they send Alzheimer’s/dementia tests to reference/referral labs versus performing them in-house, while one noted how they have plans to reinstate this testing in their lab.
Clinical reasons for testing
As MLO reported in May 2025, nearly all Americans think early Alzheimer’s diagnosis is essential, according to a survey conducted by Alzheimer's Association.1 The trend toward early Alzheimer’s/dementia detection and diagnosis was seen in MLO’s SOI survey as well, with 39% of lab professionals citing differential diagnosis as the primary clinical reason for ordering this testing and 37% citing early detection as the main reason.
Additionally, 15% of survey respondents reported monitoring progression and 9% research as the primary clinical reasons for clinicians ordering Alzheimer’s/dementia testing.
Zivjena Vucetic, MD, PhD, Chief Medical Officer (CMO) Beckman Coulter Diagnostics, shared her thoughts on the importance of testing for early diagnosis:
“Alzheimer's disease (AD) is a very complex disease where early diagnosis may change the trajectory of the disease by allowing for interventions to slow cognitive decline and improve quality of life. With the number of people over 60 worldwide expected to double by 2050, the development of blood-based AD tests is a global priority.”
Biomarkers tested
Most lab professionals surveyed (63%) reported that their labs test no specific biomarkers for Alzheimer’s/dementia testing. The most tested biomarkers were phosphorylated tau (18%) and APOE genotyping (17%). This was followed by total tau (14%), Aβ42 (12%), and neurofilament light chain (8%). Furthermore, 14% of lab professionals indicated they test other biomarkers, but these were not specified in the survey comments.
Dr. Maria-Magdalena Patru, Scientific Partner for Neurology at Roche Diagnostics, commented on the availability of Alzheimer’s disease (AD) biomarker tests for early detection of amyloid pathology and amyloid-targeting therapies which slow down cognitive decline. She highlights the central role clinical laboratories can play in translating these advancements in clinical practice.
“Evidence indicates that the use of AD biomarkers in the clinical context improves diagnosis accuracy, especially in early stages, when therapies are most effective,” said Dr. Patru. “Currently, the CSF ratio assays are an option for determining patient eligibility for therapy. Once FDA cleared blood-based biomarker tests are integrated in clinical practice, they will complement, but not entirely replace, the need for the CSF ratio assays in confirming AD pathology.”
“By validating scalable AD biomarkers tests, laboratories can address the need for greater access to diagnostics, which in turn facilitates timely therapeutic interventions,” she added. “It is expected that the combination of biomarker tests, early diagnosis, and therapy will positively impact AD patients and their families.”
Dr. Vucetic highlighted the need for testing that is applicable across diverse populations, stating:
“To develop accurate, reliable tests, we examine different biomarker levels across different populations. AD's complex nature, with variations in genetic makeup and progression, means new blood-based tests must consider heterogeneity in biomarker levels across ethnicities and races. Global partnerships with organizations like Davos Alzheimer’s Collaborative and Global Alzheimer’s Platform Foundation are essential for accessing diverse patient cohorts. These efforts aim to create equitable and effective diagnostic solutions, ensuring no Alzheimer's patient is left behind.”
Respiratory testing trends
Nearly half of laboratory professionals surveyed (48%) have seen a moderate increase in respiratory testing over the past 12 months, while nearly one-third (27%) have experienced a significant increase and nearly a quarter (24%) have seen no increase in this testing category.
Respiratory viruses tested
When asked which respiratory viruses have yielded the most positive test results, influenza A/B topped the list with 82% of survey respondents, followed by COVID-19 at 63%. Over one-third of lab professionals reported having the most positive test results for respiratory syncytial virus (RSV), 25% strep A/B, 13% rhinovirus, and 5% pneumonia.
A small percentage of those surveyed (3%) indicated they did not know which respiratory virus tests had the most positive results in their labs, and 5% selected “other” as their response, citing high volumes of positive testing results for tuberculosis and mycoplasma (local outbreak).
According to Leonard Scinto, MS, MPA, DLM(ASCP), Technical Consultant at Advancing Health Laboratories, based in South Florida, the past 12 months have been “uneventful for any significant respiratory trends” in the state’s respiratory virus activity for Influenza A/B and COVID-19. He stated:
“Influenza test positivity did not show any dramatic spikes compared to 2023-2024. The same trend was seen for COVID-19 over the past 12 months; no dramatic increases were seen.”
“RSV showed a different picture in Florida,” he added. “Florida’s RSV season is longer than the national average, starting in April and peaking in November. November 2024 showed significantly higher RSV test positivity rates than previous 2023-2024 season. This significant positive increase related to three factors: Increased RSV circulation in Florida’s elderly population, increased use of multiplex PCR panels now including RSV targets, and a change in the Florida DOH mandatory reporting of RSV testing which began in 2024.”
The director of a medical laboratory based in Maryland commented on the respiratory virus trends in her area, stating:
“We have seen an increase in enterovirus/rhinovirus testing with a significant decrease in flu and COVID-19 testing. The use of molecular respiratory panels proved to be important for the clinical treatment of respiratory infections.”
Test modalities
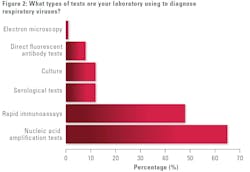
Turning to test modalities to diagnose respiratory viruses, highest on the list was nucleic acid amplification test, with 65% of survey participants citing how they used this method in their labs. Nearly half of lab professionals reported using rapid immunoassays (49%). Lower on the list were cultures (13%), serological tests (13%), direct fluorescent antibody tests (8%), and electron microscopy (1%).
Edmund Janoszewski, Senior Product Manager, Diasorin, spoke to trends in respiratory test modalities, stating:
“The emergence of respiratory testing patterns over the past two to three years has prompted many laboratories to develop integrated testing algorithms that incorporate a variety of respiratory testing methods, including rapid antigen tests, point-of-care molecular testing, targeted molecular assays performed in the lab, and flexible syndromic assays. These testing algorithms, based on diagnostic stewardship, aim to accurately identify the causative pathogens, ensure timely results to guide patient treatment, and promote effective management of healthcare costs.”
“In simpler terms, laboratories are focused on choosing the right test for the right patient at the right time,” Janoszewski continued. “Additionally, as the customer demand for accurate, fast, and affordable tests increases, diagnostic testing companies must carefully design their tests and overall testing portfolios to support the dynamic needs of expanding healthcare systems.”
Galit Gelman, Senior Commercial Marketing Manager, SEKISUI Diagnostics, commented on the benefits of combination testing for multiple respiratory viruses, stating:
“Clinicians are choosing carefully how to test for common respiratory illnesses, like Flu A&B and COVID-19, as it can have an impact on the experience their patients have while seeking answers when visiting the clinic. Long-wait times, multiple swabs, and leaving without answers can be frustrating to patients. A benefit of a point-of-care COVID/Flu combo test is a reduction to the discomfort and waiting and can really make a difference for your practice. This is why we are seeing more clinicians opt-in to molecular and antigen combo testing when Flu and COVID-19 are co-circulating.”
Medical lab supply chain strategies
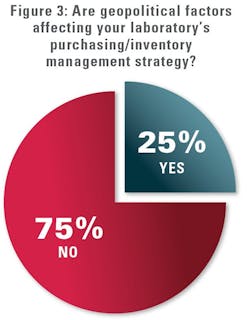
MLO also queried medical lab professionals on whether geopolitical factors were affecting their labs’ purchasing/inventory management strategies. Most (75%) said “no” and the remaining 25% said “yes.” Those responding in the affirmative were asked to elaborate on the impact.
Several survey participates commented on challenges accessing products manufactured in China, with one lab professional noting how a vendor asked their lab to switch to an alternative product manufactured elsewhere.
Others cited funding restrictions impacting their purchasing/inventory management strategies, including lack of access to capital budgets, government cutbacks and uncertainty around grants limiting their budgeting and buying ability. One lab professional commented on how it is too soon to tell how geopolitical factors will impact the medical lab supply chain but added that their lab’s GPO cited these factors as one of their top three concerns.
Scinto spoke about the uncertainties ahead, stating:
“Currently, the impact has not been felt in supply chain disruption or significant cost increase in our general lab supplies, testing reagents and media. However, this can change in a matter of weeks to months as we get more clarity on the impact trade tariffs in the U.S. and other global countries will have on specific laboratory testing supplies.”
He recommends lab leaders take an immediate proactive approach to plan for potential supply chain disruptions and testing supply cost increases in three areas:
- Identify the manufacturing country of origin. Engage laboratory supply vendors in an open, evidence-based discussion on the materials being purchased in regard to manufacturing country of origin, changes in vendor manufacturing sites and manufacturing delays due to changes in production line modifications.
- Review current vendor agreements and contracts. Review agreements for price adjustment terms. What are pricing increases tied to: USA Consumer Price Index (CPI), International Bond Rates or some obscure Global Metrix where the manufacturer is based. Are price increases annual, or can they be adjusted multiple times over 12 months? When does the agreement expire, in the next 3-5 months or 3-5 years? What will be the financial impact to current and future budget expenses and revenue margins? If you’re engaged in current contract negotiations, consider tariff protection and non-financial penalty exit clauses in new agreement terms.
- Maximize group purchasing organization (GPO) participation. Is your organization participating in a GPO that maximizes laboratory supply cost savings? Have you aligned your laboratory supply purchases through the GPO, or do you have legacy local vendor contracts? GPO participation often insulates against price increases for the term of the agreement.
How can vendors help?
MLO asked the laboratory professionals who contributed commentary for this article how manufacturers of testing equipment and supplies could better meet their needs.
“I would like to see our diagnostic manufacturers establish FDA approval for residual patient samples use,” said Scinto. “Manufacturers should seek FDA clearance for residual sample use by validating residual sample stability and performance especially for multiplex PCR. This could greatly enhance patient care, allowing reflexive test orders by clinicians on the primary/initial collection sample negating the need for patient recollection.
“This would advance both diagnostic and antibiotic stewardship,” he added. “It is estimated that over 51% of antibiotic use in poorly defined respiratory illnesses is inappropriate. Diagnostic testing through defined reflexive testing algorithms could become focused on local/regional viral prevalence. Leading to overall reduced healthcare costs and accelerated patient outcomes.”
When asked what was on her “wish list” in the realm of testing, the Maryland-based medical laboratory director stated: “We would like to see lower costs for the reagents and analyzers used in the outpatient settings.”
Reference
1. Brady E. Americans' attitudes toward Alzheimer’s testing. Medical Laboratory Observer. May 9, 2025. Accessed June 27, 2025. https://www.mlo-online.com/disease/news/55289310/americans-attitudes-toward-alzheimers-testing.
About the Author

Kara Nadeau
has 20+ years of experience as a healthcare/medical/technology writer, having served medical device and pharmaceutical manufacturers, healthcare facilities, software and service providers, non-profit organizations and industry associations.