More than 500,000 children in the U.S. between the ages of one and five are living with a blood lead level over the current U.S. Centers or Disease Control and Prevention (CDC) reference level of 5 μg/dl. Lead exposure is a significant, yet preventable, health threat to children. Exposure to lead may cause damage to the brain and central nervous system, slowed growth and development, learning and behavior problems, and hearing and speech problems.
National pediatric testing guidelines
In 2012 the CDC recommended that families with children whose blood lead value is above 5 μg/dl receive a confirmatory test as well as education on lead exposure, environmental investigations, and ongoing medical monitoring. In conjunction with the CDC recommendations, the Centers for Medicare and Medicaid Services (CMS) and the American Academy of Pediatrics recommend testing at one and two years. Experts state that testing should continue after age two for at-risk children.
Diagnostic testing methods
Clinical diagnosis of lead poisoning is difficult because exposed children are often asymptomatic, and when symptoms do exist they are nonspecific. Consequently, the only way to know if a child or adult is exposed to lead is to test. The three most commonly used methods for testing blood lead levels are inductively coupled plasma mass spectrometry (ICP – MS), graphite furnace atomic absorption spectrometry (GFAAS), and anodic stripping voltammetry (ASV):
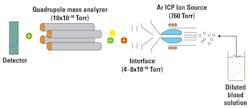
1. ICP-MS (Figure 1) is a multi-element technique that uses an inductively coupled plasma (a very high temperature ionized gas composed of electrons and positively charged ions) source to atomize the sample and subsequently ionize the atoms of interest. Sample dilution is necessary, so this requires highly skilled laboratory personnel and a highly complex CLIA registration. The limit of detection is in the range of .05 to .20 μg/dl. Throughput is estimated between 80 to 90 samples per day.
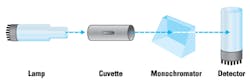
2. GFAAS (Figure 2) uses an electrically heated graphite tube to vaporize and atomize the analyte at temperatures up to 3000°C before its detection. This method is CLIA registered high complexity, requires trained laboratory personnel, and has a throughput of 120 to 300 samples per day. The limit of detection is in the order of 1 to 2 μg/dl.
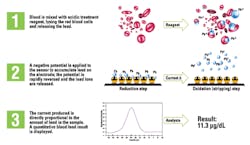
3. ASV (Figure 3) is used primarily for the determination of heavy metals. In this technique preconcentration is caused by reduction of the metal ion to the elemental state, and the stripping step is accomplished by a positive potential scan that gives an anodic current when the preconcentrated metal is oxidized. This technology is available as a moderately complex clinical lab instrument as well as a point-of-care (POC) CLIA Waived device.
The ASV moderately complex devices return results in three minutes and are available in either single-channel or six-channel options depending on laboratory workflow needs. Throughput for the single-channel system is 15 to 20 samples per hour, while the six-channel system can run approximately 90 samples per hour. They are easy to use but do require trained laboratory personnel to operate. The reporting range on these systems is 1.9 to 65 μg/dl. Both analyzers accept capillary or venous samples.
POC methods
The CLIA Waived POC device using ASV technology can run either capillary or venous samples, but most often a provider collects a finger stick sample, which is most beneficial when testing an infant or child. Trained laboratory personnel are not required to run the device, which means it is a good fit for use in physician offices, federally funded clinics, mobile health units, and other nontraditional sites. The POC reportable range is 3.3 to 65 μg/dl. If a result of 5 μg/dl is registered then, a venous sample is drawn and run using a reference method for confirmation.
The growing recognition that disease prevention is enhanced by screening for risk factors and adoption of early intervention strategies is leading to an increased use of testing for primary prevention in asymptomatic individuals. Many hospitals and healthcare systems have chosen to take advantage of the technology improvements in POC devices especially when screening for the presence of lead in asymptomatic or high-risk populations.
The value of the POC device is threefold. First, even when a blood test is ordered, up to 38 percent of patients don’t get blood tests done due to inconvenience or costs. Having the test available at the POC improves compliance. Second, having a result at the time of the provider/patient visit speeds clinical decision making, so there is no delay in treatment or other public health services. Last, patients discussing their test results with a healthcare provider are more likely to follow the recommended treatment.
“Two by two”
To date, there is no evidence that there is a “safe” lead level in a child’s blood. In the U.S., blood lead levels have continued to decline; however, the need for ongoing testing and surveillance remains. Beyond lead in paint, there are many sources for lead: soil, imported toys, pottery with leaded glaze, alternative medicines, imported candy, costume jewelry, drinking water, and second-hand smoke. Some occupations or hobbies can leave lead dust on shoes or clothing.
Clinical diagnosis of lead poisoning is difficult. Diagnostic testing is the only way to know for sure. There are multiple methods that can be used to detect lead exposure. The biggest value to children, families and our society is gained through testing done at the recommended frequency: two tests by age two.
Amy Winslow is the president and CEO of Magellan Diagnostics, the makers of LeadCare II, LeadCare Plus, and LeadCare Ultra analyzers.