It is estimated that the United States consumes approximately 75 percent of the world’s prescription drugs, and a staggering 52 million people over the age of 12 have used prescription drugs for nonmedical purposes in their lifetime.1 As more and more people become addicted to prescription pills, it is no surprise that an increase in illicit drug abuse has also been observed. For example, recent data indicate that there is a link between prescription opiate addiction and heroin abuse because the two substances target the same receptors. The link is so strong that approximately 75 percent of patients addicted to prescription opioids switch to heroin—because it is less expensive.2This has resulted in a 63 percent increase in heroin use since 2002.3 The large usage and addiction rates for both substances has created increased demand for drug screening.
“Dilute and shoot”
The growing demand for opioid screening and the increasing number of abused drugs in general puts pressure on labs to develop new techniques and increase throughput. This can pose both qualitative and quantitative challenges for analysts. Many labs are turning to a “dilute and shoot” LC/MS/MS approach when analyzing urine samples, because this technique ensures that drug compounds are not lost during the preparation procedure. But it can also cause many negative downstream effects, which can lead to increased cost per sample due to maintenance and downtime. The root cause of these issues can often be attributed to the hydrolysis procedure, which is required when performing urine analysis.
In a urine sample matrix, drugs are often present as a glucuronidated metabolite. It is standard practice to remove the glucuronide moiety prior to LC/MS/MS analysis using a hydrolysis procedure to ensure that the drugs are accurately confirmed and quantified. Hydrolysis can be performed in several ways; enzymatic hydrolysis using ß-glucuronidase is often preferred because it does not require the use of harsh acids and is a rather simple procedure. While simple, however, the procedure does introduce a new component to the sample: an enzyme. Enzymes are protein molecules that, when injected onto an HPLC column or into a mass spectrometer, can reduce sensitivity, clog columns, and build up on the mass spec source. This will eventually lead to required maintenance and subsequent downtime.
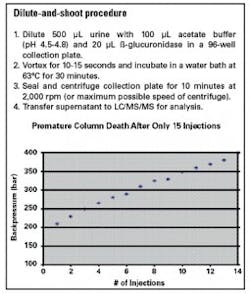
There are several methods to remove the enzyme prior to analysis, but most of these methods include more targeted sample preparation procedures that require method development such as solid phase extraction (SPE). So, despite its limitations, many labs still prefer simple sample preparation methods such as “dilute and shoot.” An optional centrifugation procedure can be performed to remove the ß-glucuronidase enzyme, but removal is often not complete. Incomplete removal of the enzyme will eventually increase backpressure (Figure 1), reduce sensitivity, and cause problems with the mass spec instrument.
ß-glucuronidase: an “out of the box” approach
Historically, protein precipitation has been favored as a go-to preparation technique for samples that are protein-rich, such as plasma. Urine does not typically contain significant amounts of protein and would not usually benefit from a protein precipitation procedure. However, urine that has been subjected to enzymatic hydrolysis with ß-glucuronidase contains a significant amount of enzyme, which by definition is a protein.
Recently, screening labs have been applying a protein precipitation technique to remove the enzyme and ensure minimum wear and tear on the column and instrument. The technique is both rapid and simple, requiring about five minutes plus dry-down time and virtually no method development. Protein precipitation is also effective, resulting in >33x increase in HPLC column lifetime and a significant reduction in backpressure (Figure 2) and mass spec downtime for maintenance. Because the technique is non-selective, it removes only proteins (ß-glucuronidase) from the sample without risking the loss of any drug compounds that may be present.
As the link between prescription and illicit drugs increases, labs must ensure that their testing procedures are able to accurately detect a wide variety of unknown compounds. These methods need to be fast and simple while not adding to downtime and maintenance, particularly in high-throughput settings. To address this challenge, labs can look outside of their traditional tool box and incorporate a cleanup technique that is not typically performed in drug screening labs. By implementing a rapid protein precipitation technique that has historically been used to clean up plasma samples in pharmaceutical and clinical labs, analysts can easily remove the ß-glucuronidase from their samples without risking the loss of drug compounds. The technique also significantly increases HPLC column lifetime and reduces mass spec maintenance requirements without the need to perform additional method development.
References
- National Institute on Drug Abuse (NIDA). Popping pills: prescription drug abuse in America. http://www.drugabuse.gov/related-topics/trends-statistics/infographics/popping-pills-prescription-drug-abuse-in-america. Accessed September 2, 2015.
- American Society of Addiction Medicine, Opioid Addiction Disease 2015 Facts & Figures. http://www.asam.org/docs/default-source/advocacy/opioid-addiction-disease-facts-figures.pdf?sfvrsn=2#search=%22opioid%20addiction%20facts%20and%20figures%22. Accessed September 2, 2015.
- Popovich, Nadja. Rapid rise of heroin use in US tied to prescription opioid abuse, CDC suggests. The Guardian, July 8, 2015. http://www.theguardian.com/us-news/2015/jul/08/heroin-use-overdose-deaths-us-prescription-opioids. Accessed September 2, 2015.
Opiate addiction is spreading and becoming more complex
The growing availability of heroin, combined with programs aimed at curbing prescription painkiller abuse, may be changing the face of opiate addiction in the United States, according to sociologists.
While heroin abuse is still relatively rare, the use of the drug is not only increasing, but it is now being coupled with the abuse of prescription painkillers, says Shannon Monnat, PhD, assistant professor of rural sociology, demography, and sociology at Penn State. She adds that the heroin-prescription drug combination is also hitting groups that were not traditionally viewed as widespread opiate users.
“One of the things we’ve found is that the simultaneous use of heroin and prescription painkillers together has increased dramatically among whites and especially among young white men,” says Monnat. She describes the recent trend as a domino effect of addiction that began in the 1980s and 1990s when the over-prescription of painkillers led to an increase in addiction to those drugs.
“Over the last several years there have been more restrictions put in place, including prescription-drug monitoring programs and the introduction of a tamper-proof opioid, making it difficult to crush, liquefy and inject the substance,” says Monnat. “What this has done is restrict access to prescription painkillers for people who previously became addicted to them. These people sometimes transition into heroin, which has become incredibly cheap and easily accessible.”
Some addicts who were introduced to heroin also turn to abusing both painkillers and heroin at the same time. While most opiate addicts are still addicted to only painkillers, the number of addicts using heroin and the number of users who are addicted to both painkillers and heroin are increasing faster than painkiller-only abusers.
The three groups of opiate abusers are distinct demographically, socioeconomically, and psychologically, Monnat adds. While heroin abuse is typically characterized as being a problem in low-income areas with high unemployment, an increasing number of heroin and painkiller-heroin addicts are employed and live in rural and small urban areas.
-MLO Staff