Point-of-care-testing takes center stage to meet demand for COVID-19 diagnostics
Many experts say that a global pandemic was inevitable. Amid cuts in funding, public health laboratories do their best to be prepared to manage unpredictable global emergencies. But in spite of these efforts, management of the novel SARS-CoV-2 has been less than ideal, and the virus continues to raise challenges and create questions.
Laboratories of all types have had to be nimble and adapt “on the fly” in order to manage the changes in testing volumes; both the drop in non-urgent testing, and the pressing demand for more COVID-19 testing. Point-of-Care Testing (POCT) is being touted as a crucial part of an integrated solution, and laboratory professionals are needed to ensure those tests are performed properly and results are interpreted with knowledge about the testing limitations.
Off to a rocky start
Right out of the gate, there were struggles with COVID-19 laboratory testing. From the beginning, testing was roadblocked by contamination issues with a RT-PCR test from the Centers for Disease Control and Prevention (CDC). Early bungling of testing efforts gave the virus time to infect thousands of people and limited testing exacerbated distancing and quarantine efforts.
Once laboratories had permission to develop tests and the U.S. Food and Drug Administration (FDA) relaxed regulations for commercial assay developers, testing options began to explode with varying degrees of efficacy. From there, laboratories faced shortages of reagents, collection swabs, and personal protective equipment (PPE).
While laboratories faced an initial drop in testing volumes for routine testing, they simultaneously faced the dire need for rapid COVID-19 testing. Many laboratories are still struggling to ramp up testing and speed turnaround times to help diminish the spread of COVID-19.
Several factors continue to increase the demand for testing, such as:
- increasing spread of the virus
- testing for preoperative patients
- testing in federally qualified health centers, nursing homes, and prisons
- orders from drive-through community events
- organizations bringing employees back to work
- universities bringing students and faculty back to campuses
Slow turnaround times delay contact tracing
Supply chain bottlenecks and staffing limitations have resulted in long turnaround times (TATs) at a time when rapid results are imperative to perform contact tracing and slow disease progression. Test results for the novel coronavirus often take so long that the results are useless in efforts to control the disease. An additional concern is that slow TATs discourage people from getting tested and practicing social distancing.
Some testing sites are struggling to provide results in five to seven days. Others are taking even longer. Long TATs are making it impossible for the United States to replicate the strategy used by other countries to effectively contain the virus – test, trace, and isolate.1 Delays in testing mean public healthcare workers are not able to notify the contacts of people who test positive. As a result, people continue to spread the virus without knowing they are infected. Anthony Fauci, MD, Director of the National Institute of Allergy and Infectious Diseases (NIAID), explained in a July podcast interview with The Wall Street Journal, “If you’re going to do contact tracing and the test comes back in five to seven days, you might as well not do contact tracing because it’s already too late.”2
According to a study performed at Harvard University, 4.3 million people need to be tested daily to gain control of the U.S. outbreak and begin to get back to normal. As of June, only 16 states had reached this mitigation benchmark, and of those, only four were doing enough testing to suppress the virus.3
Overcoming barriers and addressing challenges
As always, top laboratories find ways to address patient and public health needs, overcoming barriers to the best of their ability. Many laboratories have opted for an integrated approach, adopting multiple testing platforms to alleviate supply shortages and keep up with testing volumes. With a combination of different platforms and vendors, laboratories can ramp up testing capacity and help address supply chain issues. For example, laboratories in Alaska have managed to overcome COVID-19 testing obstacles, resulting in Alaska having the lowest number of COVID-19 deaths per capita in the United States.
A local manufacturer has been selected to deploy 3D-printed collection swabs to address the shortage, and testing is being performed in Alaska’s own public health laboratories. In addition, they have embraced the rapid TAT of POCT, are distributing these devices, and training operators in order to mitigate disease spread.4 Without a centralized federal testing plan, Alaskan officials put together an effective testing program that helped track the outbreak. Their prompt action and focus on the distribution of testing devices and end-operator training allowed them to stand up testing and tracing before the virus could rapidly spread.
Rapid POCT in the spotlight
The need to quickly act to lessen the virus’ deadly impact is making fast TAT the number one priority in COVID-19 testing. When speed of results is vital, such as in a worldwide pandemic, POCT becomes imperative. Even a less-sensitive result is significantly better than taking forehead temperatures in a doorway as people enter a building.
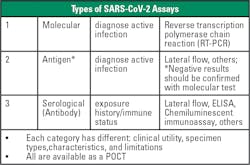
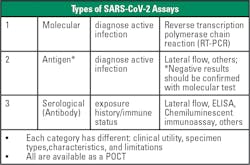
There are now numerous POCT options available for COVID-19 testing, including PCR, as well as antigen and serological tests (see Figure 1). Both molecular and antigen tests can be used for diagnostic purposes. Molecular tests use nucleic acid amplification technology to detect viral nucleic acids in SARS-CoV-2’s RNA. Molecular testing typically has the highest sensitivity and specificity. Antigen tests detect nucleocapsid antigens that are present in the respiratory system during an active infection. Antigen tests typically have high specificity but lower sensitivity in comparison to molecular tests, lending to a greater number of false negative results.Antibody testing is not yet widespread, and researchers do not know if the presence of antibodies infers immunity. Going forward, widespread use of antibody tests could help determine how much of the population has had the virus, but the tests cannot detect cases in the early stages of disease. For each of these testing methodologies, results must be interpreted alongside patient clinical information and symptoms.
The rapid introduction of so many tests to the market, the need for proper collection techniques, and concerns about lower sensitivity and specificity set the stage for laboratory professionals to ensure testing is performed appropriately. Herein lies an opportunity for laboratory professionals to explain the proper use of POCT and ensure that samples are being collected and results are being interpreted correctly. While the lesser sensitivity and specificity of POCT is acknowledged as an issue, its rapid TAT is a worthwhile trade-off in patient scenarios where speed is of the essence.
POCT for vulnerable populations
Elderly patients residing in skilled nursing facilities and nursing homes are a particularly vulnerable population. To help mitigate disease spread in this group, the Department of Health and Human Services (HHS) has begun distributing FDA-authorized POCT diagnostic devices to nursing homes in COVID-19 hotspot areas to facilitate on-site testing for patients and staff. This effort is intended to augment their current capacity for testing and improve their ability to quickly respond and prevent disease spread.
“Access to rapid point-of-care testing in nursing homes will further protect our Nation’s most vulnerable patients,” said ADM Brett P. Giroir, MD., Assistant Secretary for Health at the Department of Health and Human Services (HHS), in a press release. 5
Also, to protect vulnerable populations, HHS has awarded $760 million to purchase the Abbott BinaxNOW COVID-19 Ag Card, which costs approximately five dollars per test and provides results in about 15 minutes. The test includes a smartphone app that allows patients to receive their test results.6
The laboratory’s evolving role
The rapid spread of COVID-19 across the world has exposed major gaps in the abilities of most countries to respond to a virulent new pathogen. Rapid, reliable testing is crucial to understanding the spread of the pandemic and to mounting an appropriate response. Moving forward, as we work to control the pandemic and plan for the future, a key lesson is that early availability of diagnostic testing is of great value for patient management and public health. Thus, the development, validation, scale-up in manufacturing, and distribution of diagnostic tests must be a key priority in early preparation during an emerging infectious disease outbreak.
Rapid, integrated laboratory testing that enables quick contact tracing and immediate quarantine is part of the solution to help quickly reduce the spread of the COVID-19 virus. POCT is recognized as a key component to address the TAT challenges and facilitate effective contact tracing. While POCT is imperfect, when performed as intended, its rapid TAT is a worthwhile trade-off in certain patient care situations.
However, the complexities and limitations of POCT require knowledgeable and diligent oversight by the laboratory to ensure reliable results. Laboratory professionals who have training and knowledge about testing methodologies and limitations are well-suited to help determine when and where POCT is appropriate and to help ensure that collection and testing is being performed accurately. Medical laboratory scientists should embrace POCT as a powerful tool in the right places when speed is critical and utilize their knowledge of POCT as an opportunity to showcase their contribution to the healthcare team.
References
- Weiner R., Wan W., Hauslohner A. Long delays in getting test results hobble coronavirus response. The Washington Post. https://www.washingtonpost.com/health/long-delays-in-getting-test-results-hobble-coronavirus-response/2020/07/12/d32f7fa8-c1fe-11ea-b4f6-cb39cd8940fb_story.html Published July 12, 2020. Accessed September 17, 2020.
- Krouse S., Terlep S,. Grossman M. Covid-19 Test Results Take Longer as Infections Rise Sharply. The Wall Street Journal. https://www.wsj.com/articles/wait-times-grow-for-covid-19-test-results-as-infections-rise-sharply-11594287001. Published July 9, 2020. Accessed September 17, 2020.
- Stein R. As Coronavirus Surges, how much testing does your state need to subdue the virus? NPR. https://www.npr.org/sections/health-shots/2020/06/30/883703403/as-coronavirus-surges-how-much-testing-does-your-state-need-to-subdue-the-virus. Published June 30, 2020. Accessed September 17, 2020.
- Patterson S. Alaska built one of the most comprehensive Covid-19 testing operations in U.S. The Wall Street Journal. https://www.wsj.com/articles/alaska-built-one-of-the-most-comprehensive-covid-19-testing-operations-in-u-s-11599930000. Published September 12, 2020. Accessed September 17, 2020.
- Trump Administration announces initiative for more and faster COVID-19 testing in nursing homes. https://www.hhs.gov/about/news/2020/07/14/trump-administration-announces-initiative-more-faster-covid-19-testing-nursing-homes.html. Department of Health and Human Services. Published July 14, 2020. Accessed September 17, 2020.
- Trump Administration will deploy 150 million rapid tests in 2020. https://www.hhs.gov/about/news/2020/08/27/trump-administration-will-deploy-150-million-rapid-tests-in-2020.html. Department of Health and Human Services. Published August 27, 2020. Accessed September 17, 2020.
About the Author

Kim Futrell, MT (ASCP), MSHI
is the Products Marketing Manager at Orchard Software. Prior to joining Orchard in 2012, Futrell, who has a Master of Science in health informatics, worked as a lab manager for more than 20 years.