Biomarker technology in cervical cancer screening improves risk stratification
Cervical cancer, a preventable disease and curable if detected early, is the second leading cause of cancer death in women 20 to 39 years of age in the United States, causing 10 premature deaths per week in this age group.1 Human papillomavirus (HPV) is the single most important etiological agent, with 99 percent of cervical cancers caused by a persistent HPV infection.2 Skilled laboratorians using newer screening tests, such as HPV, in combination with Pap cytology, can provide meaningful information about a woman’s risk of disease. However, some results may contribute to a diagnostic dilemma for clinical practice.3 As biomarker technology in a triage test continues to evolve, so does the science of cancer protection and prevention.4,5 Next-generation cytology testing using objective p16/Ki-67 biomarkers delivers greater sensitivity and offers the medical lab a simplified approach for triage that improves risk stratification and provides more definitive guidance in individual patient management.6
Critical role of the lab in cervical cancer screening and diagnosis
The clinical lab is at the center of any hospital or healthcare system and contributes to greater than 70 percent of medical diagnoses and decisions made by physicians each year.7 In cervical cancer screening and diagnosis, the lab, cytologist, and pathologist have critical roles in both identifying cellular changes indicative of abnormalities (or transforming disease) and providing clinicians with evidence-based information to determine next steps for the patient.8,9
Screening programs have evolved
In the United States, cervical cancer screening programs have evolved from the Papanicolaou (Pap) cytology test first introduced in 1941. Today, the Pap is used alone or in combination with high-risk HPV DNA testing (co-testing).10 There is also an option for high-risk HPV DNA screening as the primary test.10 These developments have dramatically reduced cervical cancer incidence and deaths but some challenges remain. Not every HPV-positive woman will develop cervical cancer.11 Additionally, discrepant results may occur in co-testing where a result can be HPV-positive but have normal cytology.3 In these cases, following clinical guidelines by having the patient wait for up to a year before retesting may leave clinicians concerned about losing the patient to follow-up. Waiting may also create anxiety for the patient.
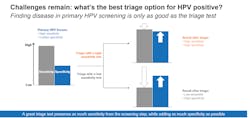
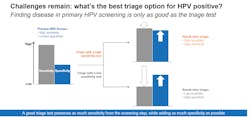
An effective triage step is required to identify who is truly at risk for disease and who is highly likely to self-resolve their infection in order to minimize the potential for under- or over-treatment. Both carry consequences. Over-treatment may result in unnecessary colposcopy and biopsies. Colposcopy is a common and relatively safe procedure. However, evidence suggests that undergoing colposcopy after an abnormal cervical cancer screening result may have a negative impact on a woman’s psychological and sexual health. Potential consequences of under-treatment are missed diagnosis with progression to more advanced disease, leading to costs and undue suffering that could have been avoided if less aggressive intervention occurred earlier.
Disease may go undetected if a highly sensitive screening test, such as hrHPV, is followed by a lower sensitivity triage test, such as Pap. An effective triage test preserves as much sensitivity from the screening step and adds as much specificity as possible to find most of the women with cervical disease while minimizing the risk of undetected disease. A triage test with both high sensitivity and high specificity enables more accurate risk stratification than one with lower sensitivity.
Risk stratification in triage
Risk stratification in triage enables labs to further differentiate women at risk who will benefit from immediate intervention from those who can be given more time to clear their infection without intervention before the next follow-up.11The risk of pre-cancer and cancer varies by HPV genotype. While almost all cervical cancers are associated with 14 genotypes of HPV, HPV16 and HPV18 are the two highest risk types, causing approximately 70 percent of cervical cancers and precancerous cervical lesions.12, 13 HPV16 carries the greatest risk, followed by HPV18, which accounts for approximately 13 percent of squamous cell carcinoma (SCC) as well as 37 percent of adeno/ adenosquamous carcinoma (ADC) of the cervix worldwide.12
Currently, clinicians use Pap cytology and HPV genotyping information to categorize women into different risk levels. While the current method works well in most clinical situations, there is room for improvement.
Biomarker-based technology provides more objective, definitive answers
Biomarkers and biomarker-based technology have been evolving to improve cancer diagnosis, classification of cancer genotypes, prognosis and prediction of response to therapy.4 Biomarker-based tests that yield objectively interpretable results can fill gaps left by Pap cytology when used in cervical cancer. Biomarker-based cytology testing relies on the detection of molecular changes happening within the cell, while Pap cytology relies on the more subjective interpretation of cell morphology.14
The application of biomarker technology – immunocytochemical staining for proteins to characterize cells – is useful in the diagnosis of cervical cancer. Two cellular proteins, p16 and Ki-67, have been shown to be co-expressed in HPV-infected cells that have started the oncogenic transformation process. p16 is a marker of cell cycle arrest and is overexpressed in HPV-infected cells and is an indicator of a transforming HPV infection; Ki-67 is a marker of cellular proliferation. In normal cells, these two biomarkers are never co-expressed.15 p16/Ki-67 co-expression suggests that the cell is undergoing HPV-induced oncogenic transformation and is strongly associated with established high-grade disease.16 A dual-stain positive p16/Ki-67 immunocytochemical stain result is strongly associated with established high-grade disease.17 In contrast, the absence of dual staining is more informative than a normal Pap result—with a relative reduction of 53 percent in risk of undetected disease compared with Pap (3.8 percent vs 8.0 percent)—offering reassurance that the patient is highly unlikely to progress to cancer in the next few years and can be given time to allow her immune system to clear the infection.15, 16,18 Next-generation cytology using technology that detects biomarkers p16 and Ki-67 offers an important and more objective step forward in clarity of results and improved disease detection.14,15
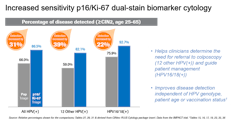
Using biomarkers to objectively look for changes on the cellular level in triage improves sensitivity by 31 percent compared to triage with Pap cytology which relies on morphological interpretation.15
Advanced technology in triage for more sensitivity, more clarity
In March 2020, the U.S. Food and Drug Administration (FDA) approved the first cervical cancer screening triage test that uses biomarker technology.6 The test uses dual-stain immunocytochemistry to simultaneously detect p16 and Ki-67 in the same cell.6 This next-generation cytology test can be performed on the same sample collected for a Pap or HPV screening test, without requiring the patient to return to the clinician’s office.6,15
Dual-stain biomarker technology provides higher sensitivity than Pap cytology triage, while maintaining high specificity.15 This combination enables more accurate risk stratification.17
Biomarker technology can also be helpful in the cervical cancer screening HPV DNA and Pap co-testing paradigm, when a woman has a positive HPV DNA result but a Pap that is negative for intraepithelial lesion or malignancy (NILM).15 A dual-stain test offers an opportunity to triage these women immediately rather than sending them back to retest in a year. Dual-stain biomarker technology identifies up to 70 percent of women with disease who would benefit from immediate follow-up.15
In its review of the new biomarker technology, the FDA considered data from the registrational IMPACT (IMproving Primary screening And Colposcopy Triage) trial, which enrolled close to 35,000 women in the United States. The study was designed to clinically validate the assay as a dual-stain triage test in different screening scenarios.6 Publication of study data is pending.
Moving closer to the goal of cervical cancer prevention
The role of HPV infection in cervical cancer has been known for decades and persistent infection is recognized as the single most important cause in almost all cases. Improved understanding of the oncogenic transformation process in HPV opens up new pathways to better identify women whose HPV infections are most likely to be associated with cervical precancer or cancer. Armed with this information, labs can confidently help clinicians in assessing risk and making decisions for the right next steps at the right time. The availability of new biomarker-based dual-stain immunocytochemistry technology can enable labs and pathologists to play an even greater role in advancing personalized medicine and answering the call for action to eliminate cervical cancer.
REFERENCES
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.
2. World Health Organization. Human papillomavirus (HPV). https:// www.who.int/immunization/diseases/hpv/en/#:~:text=Virtually%20 all%20cervical%20cancer%20cases,in%20both%20men%20and%20 women. Accessed October 17, 2020.
3. Tracht JM, Davis AD, Fasciano DN, Eltoum IA. Discrepant HPV/ cytology cotesting results: Are there differences between cytology-negative versus HPV-negative cervical intraepithelial neoplasia?. Cancer Cytopathol. 2017;125(10):795-805.
4.Selleck MJ, Senthil M, Wall NR. Making meaningful clinical use of biomarkers. Biomark Insights. 2017;12:1177271917715236.
5.Markham MJ, Wachter K, Agarwal N, et al. Clinical cancer advances 2020: annual report on progress against cancer from the American Society of Clinical Oncology.J Clin Oncol. 2020;38(10):1081.
6. Roche receives FDA approval for CINtec PLUS Cytology test to aid clinicians in improving cervical cancer prevention. Roche March 11, 2020. https://www.roche.com/dam/jcr:99f1ae3b-7e36-41f6-a2fb- 7e3af924f8da/en/roche-mediarelease-11032020-en.pdf. Accessed October 17, 2020.
7. Centers for Disease Control and Prevention. Strengthening clinical laboratories. https://www.cdc.gov/csels/dls/strengthening-clinical-labs.html. Accessed October 17, 2020.
8. Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
9. Guide for establishing a pathology laboratory in the context of cancer control. Geneva: World Health Organization; 2019. Licence: CC BY-NCSA 3.0 IGO.
10. American Cancer Society. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics. html#:~:text=Cervical%20cancer%20is%20most%20frequently,still%20 present%20as%20they%20age. Accessed October 17, 2020.
11. Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol. 2016;76 Suppl 1(Suppl 1):S49-S55.
12. Li N, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128(4):927-35.
13. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349-2359.
14. Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144(1):51-56.
15. CINtec PLUS Cytology [package insert]. Indianapolis, IN: Roche Diagnostics Corporation; 2020.
16. Yu L, Fei L, Liu X, Pi X, Wang L, Chen S. Application of p16/Ki-67 dual-staining cytology in cervical cancers. J Cancer. 2019;10(12):2654-2660.
17. Wentzensen N, Clarke MA, Bremer R, et al. Clinical Evaluation of Human Papillomavirus Screening With p16/Ki-67 Dual Stain Triage in a Large Organized Cervical Cancer Screening Program [published correction appears in JAMA Intern Med. 2019 Jul 1;179(7):1007]. JAMA Intern Med. 2019;179(7):881-888. doi:10.1001/jamainternmed.2019.0306
18. Clarke MA, Cheung LC, Castle PE, et al. Five-Year Risk of Cervical Precancer Following p16/Ki-67 Dual-Stain Triage of HPV-Positive Women. JAMA Oncol. 2019;5(2):181-186. doi:10.1001/jamaoncol.2018.4270
About the Author

Alexandra Valsamakis, MD, PhD
is Chief Medical Officer and Vice President at Roche Molecular Diagnostics.