The expanding role of biomarker testing in non-small cell lung cancer
Less than a decade since the American Society of Clinical Oncology (ASCO) endorsed routine testing for epidermal growth factor receptor (EGFR) mutations in all patients with lung adenocarcinoma, and the approval of crizotinib for the treatment of anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC) – both in 2011 – biomarker testing has become integral in day-to-day patient decisions.1 The rapid advances in precision medicine are evidenced in the continuing expansion of targeted therapeutics available and, in parallel, the elucidation of specific underlying mutations in biomarkers and the development of technology for identifying biomarkers and subtyping patients.
These new developments have improved patient survival in this leading cause of cancer death in the United States, while bringing with them the challenge of implementation in day-to-day patient care. Today, biomarker testing is vital to NSCLC subtyping and therapy selection, as organizations including the National Comprehensive Cancer Network (NCCN) and the International Association for the Study of Lung Cancer (IASLC) devote significant resources to maintaining up-to-date guidelines to help translate advances into clinical practice. This article provides an overview of how biomarker testing is used today in NSCLC, with a focus on predictive biomarkers that can guide targeted therapy.Lung cancer classification
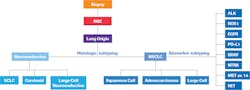
Lung cancer is the leading cause of cancer death and the most common cancer worldwide. In the U.S., the National Comprehensive Cancer Network (NCCN) estimates 228,820 new cases and 135,720 deaths in 2020. The advent of targeted therapeutics, powered by the discovery of biomarkers and underlying molecular pathways, has opened the door to improved survival. All of this requires the accurate classification of patients not only by histological subtypes but also biomarkers of oncogenic mutations and fusions and immune response to the cancer.
Non-small cell lung cancer (NSCLC) represents 85 percent of all lung cancer and has a slightly more favorable survival rate than small-cell lung cancer. During initial evaluation and diagnosis, NSCLC is further subtyped into adenocarcinoma, squamous cell carcinoma and large cell carcinoma. Adenocarcinoma is the most common, representing 60 percent of all NSCLC. In addition to histological subtyping, an NSCLC diagnosis also includes staging information.2 According to the NCCN, 55 percent of cases from 2009 to 2015 were stage IV (metastasized) at diagnosis. Many current guidelines for biomarker testing are focused on patients with metastatic NSCLC.3
NSCLC biomarkers
NSCLC biomarkers fall into two categories. Prognostic biomarkers are indicative of patient survival regardless of treatment. KRAS mutations represent one of the best-known prognostic biomarkers and are associated with shorter survival. For patients with KRAS mutations, targeted therapy is not available. Further biomarker testing may not be beneficial, with the exception of PD-L1, as immune checkpoint inhibitors (ICIs) appear to be effective.
Predictive biomarkers are associated with effectiveness of the corresponding targeted therapy and, thus, are used to identify patients who are likely to benefit from a specific therapy. This discussion will focus on predictive biomarkers.
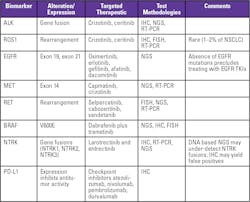
Anaplastic lymphoma kinase (ALK) gene fusions have been identified in a small subset (5 percent) of NSCLC patients who will benefit from targeted therapy such as crizotinib or ceritinib. ALK testing is commonly performed using immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). ALK fusions can also be detected using next-generation sequencing (NGS).
Current guidelines recommend all patients diagnosed with lung adenocarcinoma be tested for ROS proto-oncogene 1 (ROS1) rearrangements. Patients with ROS1 mutations respond to crizotinib or ceritinib. Given the rarity of ROS1 rearrangements (1 percent to 2 percent of NSCLC), the use of an IHC assay to detect ROS1 protein expression prior to confirmatory testing may decrease the overall cost of ROS1 testing.
In addition, current guidelines also recommend that patients diagnosed with adenocarcinoma be tested for epidermal growth factor receptor (EGFR) mutations. Deletions in exon 19 and a point mutation in exon 21 are the most common, and both are associated with sensitivity to small-molecule EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib, gefitinib, afatinib, osimertinib and dacomitinib. EGFR mutations are detected using NGS.
MET exon 14 skipping mutations occur in 3 percent to 4 percent of patients with adenocarcinomas and 1 percent to 2 percent of patients with other NSCLC histologies. Targeted therapy includes capmatinib and crizotinib. NGS and reverse transcription polymerase chain reaction (RT-PCR) assays can be used to detect METex14 skipping mutations.
RET is a tyrosine kinase receptor that affects cell proliferation and differentiation. RET rearrangements (fusions) may occur in 1 percent to 2 percent of patients in NSCLC, more frequently in patients with adenocarcinoma. RET rearrangement positive patients respond to selpercatinib, cabozantinib and vandetanib. FISH, RT-PCR and NGS assays can be used to detect RET rearrangements.
B-Raf proto-oncogene (BRAF) point mutations, specifically one associated with a change in amino acid position 600 (V600E), are found in 1 percent to 2 percent of patients with adenocarcinoma. Guidelines recommend testing for BRAF mutations in patients with metastatic nonsquamous NSCLC based on data showing efficacy of dabrafenib plus trametinib for patients with BRAF V600E mutations. BRAF mutations are tested using NGS and PCR assays.
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions (NTRK1, NTRK2, NTRK3) occur in 0.2 percent to 3.3 percent of patients with NSCLC and resulting tropomyosin receptor kinase (TRK) fusion proteins have been identified as oncogenic drivers. With the availability of selective TRK inhibitors larotrectinib and entrectinib, NCCN guidelines recommend NTRK gene fusion testing in patients with metastatic NSCLC patients, who are negative for main oncogenic driver mutations like ALK, ROS1, EGFR and BRAF. A pan-TRK IHC assay is available in addition to FISH and NGS.
Programmed death ligand 1 (PD-L1) is a protein expressed by tumor cells and tumor infiltrating immune cells to inhibit T-cell mediated cell death by binding to PD-1, a receptor expressed on activated cytotoxic T-cells. PD-L1/PD-1 binding weakens the immune system’s response to cancer. By blocking PD-L1/PD-1 interaction, immune checkpoint inhibitors (ICIs) restore T-cell activation.4 Approved ICIs include atezolizumab, nivolumab, pembrolizumab and durvalumab. In May, 2020, NCCN Guidelines were updated to specify that only patients who test positive for PD-L1 expression should be administered first-line ICI therapy, by deleting “or unknown” in its statement about PD-L1 expression levels and ICI therapy.5
Lastly, it should be noted that ALK and ROS1 fusions, BRAF mutations and sensitizing EGFR mutations are generally mutually exclusive. Thus, for patients who relapse on initial therapy based on testing positive for ALK or ROS1 fusions, subsequent targeted therapy for the other mutations listed here is not recommended.5
Selecting biomarker testing methodologies
IHC, FISH and NGS are currently the most commonly used platforms for biomarker testing. PCR is also used, but to a lesser degree. Several factors should be considered in assessing the options, with the overall goal of elevating the standard of patient care by supporting timely decisions. With this in mind, first and foremost is the quality of the diagnostic information, driven not only by the quality of test results but also by the extent to which a test result is actionable (e.g., availability of a targeted therapy). Turnaround time is important, as a test result can not only drive the treatment decision but also inform next steps in the subtyping process. Maximizing diagnostic information from limited specimen is critical, as are cost considerations.
Because of the amount of information available, NGS is a commonly used diagnostic tool for NSCLC. At 10 days to two weeks, NGS turnaround time is a challenge, but the level of information (e.g., with up to 500 markers) is unmatched, often justifying not only the turnaround time but also the cost. However, the large amount of information also demands careful interpretation before it is used to make treatment decisions.
At the opposite end of this continuum is IHC, which requires a small sample (a single tissue section), with results available generally within 24 hours and at a minimal cost. Improvements in technology and quality of results further add to the usefulness of IHC by reducing the need for confirmatory testing in some cases (e.g., in ALK testing). FISH is also a staple for biomarker testing.
The optimal use of cell-free DNA in the clinic has been a topic of interest. Proponents suggest its utility in patients who lack sufficient tissue for biomarker testing, and those who are progressing on their initial therapy and for whom a repeat biopsy is not possible.1 Another recent development is the use of tumor mutational burden (TMB) as a biomarker for patients with metastatic NSCLC and in particular for predicting response to immune checkpoint drugs independent of PD-L1 expression. However, there is no consensus on how to measure TMB.5
An additional consideration for biomarker testing is its use for therapy selection. In the U.S, approved tests are designated as either a companion diagnostic, which is required for the patient to receive the therapy, or a complementary diagnostic, which allows the clinician more discretion in prescribing the corresponding therapeutic. The designation may change as additional clinical data becomes available.
The laboratory’s role
The rapidly changing landscape of targeted therapies in NSCLC presents the laboratory with several opportunities to contribute to improved patient outcomes. As new biomarkers are identified or as guidelines are updated on the use of specific biomarkers, the laboratory and pathology can be an important resource for the oncologist in highlighting new developments and offering guidance on how they can be incorporated into day-to-day practice.
At the same time, the laboratory has the responsibility to bring new tests online as quickly as possible – not only in implementing the tests but also considering how they fit into current clinical workflow and the institution’s testing algorithms. A strategic perspective of the laboratory’s test offerings – menu, methodologies, workflow – will guide conversations with the clinical team in determining the best solutions for the patient.
References:
1. Pennell NA, Arcila ME, Gandara DR, West H. Biomarker testing for patients with advanced non-small cell lung cancer: Real-world issues and tough choices. Society of Clinical Oncology Educational Book 2019; 39: 531–542. doi: 10.1200/EDBK_237863.
2. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest 2017; 151(1):193–203.
3. Riely GL. What, when and how of biomarker testing in non-small cell lung cancer. JNCCJ 2017; 15:686–688.
4. Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019; 30:1321-1328.
5. NCCN Guidelines: Non-small cell lung cancer. Version 4.2020, May 2020.
About the Author

Bharathi Vennapusa, MD
is a pathologist on the Medical and Scientific Affairs team at Roche Diagnostics Corporation. Previously, she served as medical director for the Ventana Medical Systems CAP/CLIA laboratory and as director of clinical operations. During her tenure at Ventana, she worked on several translational and companion diagnostic IHC, ISH and multiplexing assays, as well as digital pathology algorithms.