New biofluid biomarkers in neurodegeneration
An early intervention in Alzheimer’s disease (AD) could be accomplished by having biomarkers that can reliably predict cognitive decline before the onset of clinical symptoms.1 Recently, a research framework was released by the National Institute on Aging together with the Alzheimer’s Association that emphasized the implementation of biomarkers in the diagnosis of AD. The amyloid, tau and neurodegeneration (ATN) classification system recommends the measurement of cerebrospinal fluid (CSF) biomarkers such as beta-amyloid (Aβ42) or Aβ42/Aβ40 ratio, phosphorylated tau (P-tau), and totaltau (T-tau) to evaluate amyloidosis, neurofibrillary tangles and neurodegeneration, respectively.2
However, CSF Aβ and Ptau are markers for determining the disease state and possess limited prognostic value for estimating the onset of cognitive decline or monitoring disease progression. Therefore, there is an increasing demand to identify synaptic proteins as staging biomarkers capable of predicting the onset of cognitive decline and/or the disease course.3 Furthermore, the ATN research framework recommends exploring other biomarkers for neurodegeneration, such as neurofilaments or neurogranin that can effectively predict cognitive decline.2
Phosphorylated neurofilament heavy chain
Up-and-coming star analytes in neurology are the neurofilaments (Nf). They are gaining rising attention as highly specific biomarkers for axonal damage in neurodegenerative, inflammatory, vascular and traumatic diseases. Nfs levels are largely elevated in rapidly progressing neurodegenerative diseases such as prion and motor neuron diseases (MND), as well as in frontotemporal dementia (FTD).4,5
Furthermore, many studies are looking into Nfs as potential diagnostic biomarkers for amyotrophic lateral sclerosis (ALS), since the diagnosis of this MND currently requires the description of upper and lower motor neuron dysfunction, which according to studies, causes a delay of approximately one year from symptom onset to diagnosis (Figure 1).5-9 With a median survival of three years following symptom onset, an early diagnosis would permit rapid commencement of treatment and enrollment of appropriate patients into clinical trials.10
Additionally, both NfL and pNfH similarly discriminated ALS from controls with a high positive predictive value (PPV). Furthermore, no difference in Nf levels was observed between familial and sporadic forms of the disease.9 Another two-center study confirmed the diagnostic utility and PPVs of CSF NfL and pNfH in ALS compared with controls. In this study, pNfH portrayed a significantly higher diagnostic sensitivity and specificity and a better negative predictive value for ALS compared with NfL, which was increased in other diseases such as FTD.8 Two additional studies confirmed the superior diagnostic potential of pNfH in ALS.11,12 Furthermore, Nf levels were significantly lower in ALS patients with slower disease progression and longer survival. There was a proportional increase of both pNfH and NfL levels with the number of regions and with the involvement of both upper and lower motor neurons, but the site of onset did not alter Nf levels.8
Nfs are produced in neurons and the concentration in blood is approximately 10 times lower compared to CSF.5 De Schaepdryver and colleagues evaluated the performance of pNfH in CSF and serum of ALS patients using the novel high sensitive pNfH ELISA, which was commercialized by EUROIMMUN. Good correlations were observed between pNfH levels in CSF and serum of ALS patients and both levels diagnostically differentiated ALS patients from controls. Additionally, CSF and serum pNfH concentrations correlated well with the disease progression rate.10
Evaluating pNfH in serum would be a promising tool to study the pre-diagnostic period of ALS, as well as to potentially decrease the diagnostic delay. In a follow-up study, De Schaepdryver and colleagues measured the serum pNfH levels in patients retrospectively, prior to the diagnosis of primary sporadic ALS. Serum pNfH concentrations were elevated up to 18 months prior to the diagnosis of ALS compared to healthy controls. Additionally, the pNfH levels continued to rise leading up to diagnosis (Figure 2) and an elevated serum pNfH concentration was observed to be associated with a higher risk of death.6
Neurogranin
Neurogranin is a post-synaptic protein and plays a critical role in long-term potentiation signaling in neurons. In AD, neurogranin levels are reduced in the brain, but elevated in the CSF and correlate to a lesser extent with CSF tau and amyloid.13,14 In patients with mild cognitive impairment (MCI) and AD, a good correlation was observed between increased baseline CSF neurogranin levels and rapid declines in cognition, hippocampal volumes and cortical glucose metabolism.15 Kester and colleagues evaluated the role of neurogranin in the diagnosis, prognosis and monitoring of AD. In their study, CSF neurogranin levels at baseline were elevated in AD patients compared with subjects with normal cognition.
Additionally, baseline neurogranin concentrations were elevated in MCI patients who progressed to AD, compared with those who did not worsen to dementia. Therefore, neurogranin is a promising predictive biomarker for progression of AD and elevated levels might suggest presymptomatic synaptic dysfunction.16 In another study, CSF neurogranin levels were significantly increased in patients with MCI, AD, and those with elevated T-tau levels compared with cognitively healthy subjects. Additionally, CSF neurogranin exhibited a positive correlation with CSF Ttau, but negatively correlated with CSF Aβ42/Aβ40 ratio, indicating that both CSF T-tau and neurogranin could be markers of synaptic dysfunction.14
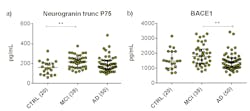
In 2016, ADx NeuroSciences designed a novel immunoassay that specifically targeted a Cterminally truncated 75 residue fragment of neurogranin (neurogranin trunc P75, hereafter referred to as neurogranin).3 This assay was later validated and commercialized by EUROIMMUN. De Vos and colleagues evaluated the added diagnostic potential of the ratio of neurogranin/betasecretase 1 (BACE1) to the classical biomarkers in AD.3 BACE1 is the transmembrane aspartyl protease that causes the production of toxic Aβ42 aggregates.17 In this study, CSF neurogranin/BACE1 ratio significantly correlated with longitudinal reductions in Mini-Mental State Examination (MMSE) scores in MCI and AD patients.
An elevated CSF neurogranin/BACE1 ratio predicted a faster decline in MMSE scores, indicating that this ratio could potentially be a marker of AD progression. Such correlations were not observed with other classical AD biomarkers such as CSF tau or Aβ, neither as single analytes nor as ratios. Furthermore, both CSF neurogranin and BACE1 concentrations increased when progressing from cognitively normal to MCI and subsequently reduced when progressing to AD (Figure 2). In short, a higher diagnostic performance was observed with the neurogranin/BACE1 ratio when compared with single analytes including CSF T-tau, Aβ42, Aβ40, and Aβ38 in AD.3 The potential value of CSF neurogranin/BACE1 ratio has been further elaborated and confirmed in early stages of AD.18
Schaeverbeke et al. showed that CSF biomarkers including neurogranin, BACE1, Aβ42 and Aβ38, as well as markers of innate immunity, correlated well with reduced gray matter volume and increased amyloid load in cognitively normal older subjects in the precuneus. Therefore, measuring biomarkers of synaptic integrity, such as neurogranin, would provide valuable information regarding the underlying neurodegenerative process in asymptomatic subjects who are at risk of progressing to AD.19
Furthermore, a study demonstrated significantly increased CSF neurogranin levels only in AD patients, but not in other neurodegenerative conditions, suggesting that CSF neurogranin, along with classical biomarkers such as the CSF P-tau and Aβ42/Aβ0 ratio, could be a useful tool aiding in the differential diagnosis of AD.20
In summary, biomarkers that can track neurodegeneration up to the level of synaptic dysfunction are of significant interest as reliable clinical endpoints in AD trials. Lately, there have been many biomarkers proposed for tracking synaptic integrity, but it is not yet understood which combination of biomarkers would provide the highest predictive value. By including biomarkers as clusters such as the CSF betaamyloids, tau, neurogranin, neurofilaments etc., it might be possible to track neurodegeneration at an early stage. These studies would provide valuable feedback regarding mechanisms of action of candidate therapeutics as different biofluid biomarkers reflect different pathophysiological processes in AD.1,19
References
- Merluzzi AP, Vogt NM, Norton D, et al. Differential effects of neurodegeneration biomarkers on subclinical cognitive decline. Alzheimers Dement (NY). 2019;5:129-138.
- Jack CR, Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562.
- De Vos A, Struyfs H, Jacobs D, et al. The Cerebrospinal Fluid Neurogranin/BACE1 Ratio is a Potential Correlate of Cognitive Decline in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. 2016;53(4):1523-1538.
- Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology. 2018;14(10):577-589.
- Poesen K, Van Damme P. Diagnostic and Prognostic Performance of Neurofilaments in ALS. Front Neurol. 2019;9:1167-1167.
- De Schaepdryver M, Goossens J, De Meyer S, et al. Serum neurofilament heavy chains as early marker of motor neuron degeneration. Annals of clinical and translational neurology. 2019;6(10):1971-1979.
- Galvin M, Madden C, Maguire S, et al. Patient journey to a specialist amyotrophic lateral sclerosis multidisciplinary clinic: an exploratory study. BMC Health Services Research. 2015;15(1):571.
- Poesen K, De Schaepdryver M, Stubendorff B, et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology. 2017;88(24):2302.
- Steinacker P, Feneberg E, Weishaupt J, et al. Neurofilaments in the diagnosis of motor neuron diseases: a prospective study on 455 patients. Journal of Neurology, Neurosurgery & Psychiatry. 2016;87(1):12.
- De Schaepdryver M, Jeromin A, Gille B, et al. Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 2018;89(4):367.
- Li D-W, Ren H, Jeromin A, et al. Diagnostic Performance of Neurofilaments in Chinese Patients with Amyotrophic Lateral Sclerosis: A Prospective Study. Front Neurol. 2018;9:726-726.
- Rossi D, Volanti P, Brambilla L, Colletti T, Spataro R, La Bella V. CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. Journal of Neurology. 2018;265(3):510-521.
- Casaletto KB, Elahi FM, Bettcher BM, et al. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology. 2017;89(17):1782-1788.
- De Vos A, Jacobs D, Struyfs H, et al. C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer’s disease. Alzheimers Dement. 2015;11(12):1461-1469.
- Portelius E, Zetterberg H, Skillbäck T, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer’s disease. Brain : a journal of neurology. 2015;138(Pt 11):3373-3385.
- Kester MI, Teunissen CE, Crimmins DL, et al. Neurogranin as a Cerebrospinal Fluid Biomarker for Synaptic Loss in Symptomatic Alzheimer Disease. JAMA neurology. 2015;72(11):1275-1280.
- Vassar R, Bennett BD, Babu-Khan S, et al. β-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science. 1999;286(5440):735-741.
- Kirsebom B-E, Nordengen K, Selnes P, et al. Cerebrospinal fluid neurogranin/β-site APP-cleaving enzyme 1 predicts cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2018;4:617-627.
- Schaeverbeke J, Gille B, Adamczuk K, et al. Cerebrospinal fluid levels of synaptic and neuronal integrity correlate with gray matter volume and amyloid load in the precuneus of cognitively intact older adults. J Neurochem. 2019;149(1):139-157.
- Wellington H, Paterson RW, Portelius E, et al. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology. 2016;86(9):829-835.
About the Author

Iswariya Venkataraman, PhD
serves as Scientific Affairs Lead, EUROIMMUN U.S. She’s currently a member of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Private Partner Scientific Board (PPSB), an independent, pre-competitive forum for study-related scientific exchange in the field of neurodegeneration. During her doctoral studies in neuroscience, she identified novel auto-antigens in autoimmune hyper-excitability disorders.

Philipp Arendt, PhD
is the Global Product Manager in neurodegenerative diseases at EUROIMMUN headquarters in Lübeck, Germany. He has a PhD in Biochemistry and Biotechnology, and he is responsible for advancing the development of customer-oriented products and global marketing and sales strategies.