sPLA2-llA—the next step to enhancing your cardiac testing panel
Earning CEUs:
For a printable version of the June CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
JUNE LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss disease and mortality statistics of cardiovascular disease (CVD).
2. Recall the new biomarker being studied for CVD and its utility in early risk assessment.
3. Describe the chemical process by which sPLA2--llA acts on the coronary artery wall.
4. Discuss other diseases that sPLA2-llA is being used on.
Cardiovascular disease (CVD) is the number one cause of death globally, with an estimated 17.7 million people dying each year. This figure represents approximately 31 percent of all global deaths.1 Worryingly, this figure is set to increase to over 23.6 million by 2030. These startling statistics highlight the urgent need for better and earlier identification of at-risk individuals; this is especially true as many CVD cases can be prevented with appropriate lifestyle changes. This article will discuss the utility of sPLA2-llA, a novel biomarker for use in CVD risk assessment.
Traditional and routinely run biomarkers for CVD risk include lipid assays such as Total Cholesterol, HDL Cholesterol (HDL-C), LDL Cholesterol (LDL-C), and Triglycerides. There is, however, a growing body of research and evidence indicating that additional risk assessment biomarkers need to be considered. Conventional risk assessment markers like those mentioned previously detect a mere 20 percent of all CVD patients. As the prevalence of CVD continues to rise worldwide, the need for reliable risk markers has never been more important.
sPLA2-llA has been proven to have clinical utility as a biomarker of inflammation. Inflammation is a process by which the body launches an attack utilizing our white blood cells. This response results in redness and swelling to either eliminate the pathogen or rid the body of an intruder.2,3 Inflammation is a common response in many disease states, in some cases the body’s immune system triggers an inflammatory response when there are no invaders to fight off. Inflammation is also associated with CVD. Although not a direct cause of CVD, inflammation is common in heart disease and stroke patients and is thought to be a sign of an atherogenic response. It is believed that in CVD the body perceives the plaque as abnormal or foreign and in response the body tries to prevent the plaque from entering the blood.3 However, in some circumstances, the plaque may rupture and come in contact with the blood triggering clot formation.3
Early risk assessment helps to reduce the risk of a cardiac event occurring. Identifying those at highest risk of CVD and ensuring they receive appropriate treatment can prevent premature death.4 Early risk assessment is particularly important in people who present with one or more risk factors including hypertension, diabetes, or hyperlipidemia. As stated before, by 2030 it is estimated that almost 23.6 million people will die from CVD, with heart disease and stroke projected to remain the leading causes of death.4 This provides further confirmation that early diagnosis is an essential step in reducing the number of individuals affected.
The financial burden that CVD places on health services makes the development of a diagnostic biomarker even more essential. sPLA2-llA, a member of the secretory phospholipase A2 family, has been found to have clinical utility as an inflammation biomarker specifically in the diagnosis of CVD risk. The addition of sPLA2-llA could compliment the labs existing cardiac risk panel, providing a different outlook and method of assessing cardiac concerns in patients.
Biological significance
The phospholipase A2 family encompasses a wide range of enzymes that are all capable of hydrolyzing the sn-2 ester bond of a natural phospholipid substrate. This reaction produces lysophospholipids and free fatty acids. Despite similar structural features, catalytic mechanisms, and evolutionary relationships, the superfamily of PLA2 enzymes is divided into fifteen separate groups and a number of subgroups.5 Representing one of the groups is the secretory PLA2 (sPLA2) family, which consists of ten catalytic active enzymes. These enzymes share the same characteristics as the PLA2 superfamily but have additional unique features.
sPLA2-llA
sPLA2-llA is the prototypic member of the group II sPLA2 subfamily and has been shown to be induced by pro-inflammatory stimuli in a wide variety of cells and tissues. It has been found to be associated with a number of inflammatory diseases including coronary artery disease and atherosclerosis.5 These factors have contributed to its nickname, “inflammatory sPLA2.”
Pro-inflammatory sPLA2-llA
sPLA2-llA can also be referred to as a bactericide and is involved in the degradation of the bacterial membrane providing a host defense mechanism against microbial infection. sPLA2-llA is highly cationic and has a higher affinity for anionic phospholipids, such as phosphatidylethanolamine (PE). This affinity means that sPLA2-llA preferentially binds to PE over phosphatidylcholine (PC). PE is a phospholipid that is abundantly found on bacterial membranes. This allows the enzyme to penetrate the cell and hydrolyze membrane phospholipids causing bacterial death.5
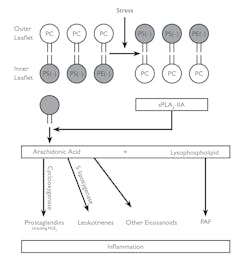
Figure 1 illustrates how sPLA2-llA binding to anionic phospholipids leads to its activation and promotes inflammation.5 The oxidation of phospholipids produces stress. This stress causes the anionic phospholipids phosphatidylserine (PS) and PE to be transported to the outer leaflet. This reaction activates cationic sPLA2-llA.
The higher activity of sPLA2-llA promotes the hydrolysis of the outer leaflet phospholipids into arachidonic acid and lysophospholipids. Through the cyclooxygenase and 5 – lipoxygenase enzymes, arachidonic acid is converted into prostaglandins, leukotrienes, and other eicosanoids. The lysophospholipids are converted into platelet-activating factors (PAFs). All four of the products produced have been linked to inflammation. Detecting the release of sPLA2-llA could therefore be a good starting point for the diagnosis of diseases related to inflammation.5
Figure 1. Inflammation5
sPLA2-llA hydrolysis reaction
sPLA2-llA production of fatty acids and biologically active phospholipids plays an important role in platelet, monocyte, and endothelial activation, processes known to be critical steps in atherogenesis.6 Unlike traditional cardiac biomarkers used to predict adverse outcomes in patients with acute coronary syndrome (ACS), sPLA2-llA has been shown to act at multiple pathways involved in atherogenesis, from lipid oxidation to modulation of vascular and inflammatory cell activation and apoptosis.7
Key observations through research found that sPLA2-llA mediated modification of lipoproteins plays a role in the development of atherosclerosis. The surface of both LDL Cholesterol (LDL-C) and HDL Cholesterol (HDL-C) is surrounded by phosphatidylcholine (PC)—a type of phospholipid which has been scientifically proven to serve as a good extracellular target for several isoforms of sPLA2-llA. sPLA2-llA works by hydrolyzing these phospholipids resulting in the production of free fatty acids and lysophosphatidylcholine (LPC) which can generate pro-inflammatory actions, accelerating atherosclerosis.6 Hydrolysis of LDL-C correlates with the production of the more atherogenic, small dense LDL cholesterol (sdLDL-C) particles. The sPLA2-llA -processed LDL-C particles contain a large amount of lysophospholipids and exhibit the property of “small-dense” or “modified” LDL-C, which facilitates foam cell formation from macrophages. Research has shown that high levels of sdLDL-C compared to less dense, larger LDL-C increases the risk of coronary heart disease.
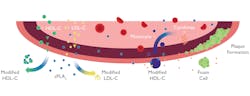
Figure 2 illustrates the proposed role of sPLA2-llA in the development of atherosclerosis. The diagram highlights the role sPLA2-llA has in the hydrolysis of LDL-C into the more atherogenic sdLDL- C.6
Figure 2. Atherosclerosis6
Comparison of sPLA2-llA with the traditional Lp-PLA2 test
Lp-PLA2 is a cardiac biomarker, sharing similarities with sPLA2-llA as it too is a member of the phospholipase A2 enzyme family. Both sPLA2-llA and Lp-PLA2 have associations with LDL-C. LDL-C carries Lp-PLA2 to the coronary artery walls where it activates an inflammatory response. This process makes plaques, if present, more prone to rupture. This enzyme is associated with causing inflammation of the coronary artery walls, indicating that high levels of Lp-PLA2 would increase the risk of a heart attack or stroke.8 In contrast, in blood, sPLA2-llA can hydrolyze LDL-C producing sdLDL-C which is highly atherogenic.9 sPLA2-llA is linked to the formation and destabilization of atherosclerotic plaques. Research has found that increased sPLA2-llA protein expression increases with atherosclerotic lesion development.
Figure 3 illustrates the process of atherogenesis and atherosclerosis and the formation of plaques. These are processes that both sPLA2-llA and Lp-PLA2 are involved in.20
Figure 3. Atherogenesis leading to the atherosclerosis20
Though involved in similar mechanisms, research has found that among biomarkers of inflammation sPLA2-llA mass improved risk discrimination for identifying patients that have an increased risk of major adverse cardiovascular events.10 The biological role of Lp-PLA2 has been controversial, with contradictory antiatherogenic and proatherogenic functions.9
Research conducted found a significant association between sPLA2-llA mass and diagnosis of CVD while the Lp-PLA2 activity appeared to not be associated with CVD diagnosis. Furthermore, patients with sPLA2-llA at presentation in the highest quartile had a statistically higher incidence of cardiac death and myocardial infarction (MI) however, no association was observed with Lp-PLA2 activity.11 Although Lp-PLA2 activity has been associated with some cardiovascular diseases, researchers question whether Lp-PLA2 has clinical utility and its role as a risk prediction biomarker.12
Relevant studies
1. Association between type II secretory phospholipase A2 plasma concentration and activity and cardiovascular events in patients with coronary heart disease (2009).13
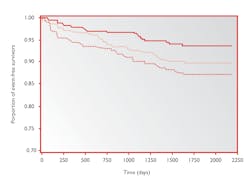
Figure 4 provides an estimation of event free survivors over a number of days depending on the level of sPLA2-llA mass present in the patient. As shown in the graph, the lower line represents the patients with the highest levels of sPLA2-llA. The proportion of event free survivors is lower for this tertile in comparison. This proportion further confirms the importance of measuring sPLA2-llA for preventing secondary coronary events.
Figure 4. Kaplan-Meier estimates of secondary fatal and non-fatal CVD events during follow-up according to tertiles of sPLA2-llA mass at baseline13
2. Lipoprotein associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress (2006).9,14
A study carried out by KAROLA involved measuring sPLA2-llA mass and sPLA2-llA activity in stable coronary disease. sPLA2-llA mass and sPLA2-llA activity showed a relatively good correlation (r = 0.63), and both measurements were significantly associated with an adverse outcome. sPLA2-llA mass appeared to perform better as a risk predictor than sPLA2-llA activity when extreme tertiles were compared.
3. Prognostic value and the changes of plasma levels of secretory type II phospholipase A2 in patients with coronary artery disease undergoing percutaneous coronary intervention (2003).9,15
A small study was carried out on patients who were angiographically proven stable coronary artery disease (CAD). Circulating levels of sPLA2-llA mass were higher in these patients than in control individuals. In addition, elevated baseline levels of sPLA2-llA were independently associated with adverse outcomes including; coronary death, MI, and coronary revascularization.
Research has shown that levels of sPLA2-llA increase immediately after mechanical disruption of the coronary artery plaque by percutaneous coronary intervention (PCI). A PCI is a non-surgical procedure that uses a catheter to place a stent in the blood vessels in order to open vessels that have been blocked by atherosclerosis. sPLA2-llA responds rapidly to the inflammatory activity in atherosclerotic arteries and plaque rupture. Also, the higher levels of sPLA2-llA after PCI could be used to predict risk of future coronary events.
Additional uses of sPLA2-llA
Scientific research has consistently highlighted the potential of sPLA2-llA as a risk marker for CVD. However, there is also a growing body of evidence to support the clinical use of sPLA2-llA as a diagnostic marker in other disease areas. Sepsis is a serious complication of infection and without quick treatment it can lead to multiple organ failure and death.16 Survivors of sepsis suffer from various complications that arise from organ dysfunction, which can result in severe impairment in their quality of life. The key to sepsis management relies on prompt and accurate diagnosis. This is because every hour appropriate treatment with antibiotics patient mortality increases by five to 10 percent. There has therefore been considerable effort among researchers to discover the most reliable sepsis biomarker.17
The current guidelines for sepsis diagnosis follows the Systemic Inflammatory Response Syndrome (SIRS) criteria. This involves monitoring the patients’ temperature, heart rate, respiratory rate, and WBC count. Several blood tests may also be employed including procalcitonin (PCT) which is the most commonly used biomarker for the diagnosis of sepsis. The accuracy and reliability of PCT however is questionable. Research was conducted to compare different biomarkers of sepsis and their accuracy in early rule in/out of sepsis. The study involved testing 168 different biomarkers (including sPLA2-llA and PCT) in pediatric and adult intensive care units. Of the 168 markers tested, only five biomarkers were found to have greater than 90 percent specificity for diagnosis, this included sPLA2-llA but excluded PCT.18
PCT is the current diagnostic and prognostic biomarker utilized in emergency departments. Although PCT and sPLA2-llA are correlated, the accuracy of PCT as a biomarker alone is significantly lower than sPLA2-llA.18
sPLA2-llA has been found to demonstrate significant association with the presence of sepsis. Studies have found that sPLA2-llA as an early marker of sepsis carries substantial clinical significance. Although further studies are required for confirmation, this provides another potential use of this biomarker. sPLA2-llA has also shown clinical utility as a biomarker for chronic inflammatory conditions such as rheumatoid arthritis.
Methodology
Randox utilizes the latex enhanced immunoturbidimetric methodology in the development of the sPLA2-llA assay. Traditionally, ELISA-based methods were developed for the detection of sPLA2-llA levels. The main downfall of the ELISA method is that it is time-consuming for practical clinical diagnostic use.19 Conversely, immunoturbidimetry is the most popular methodology as it can be easily adapted for high volume testing in automated analyzers.21
Conclusion
CVD continues to be the leading cause of death worldwide.1 Consequently, it is vital that superior assays, such as sPLA2-llA are implemented to detect the disease in its earliest stages for the early and effective implementation of treatment plans, reducing the financial burden on health care systems. As sPLA2-llA offers clinical utility as an inflammatory biomarker, specifically in the diagnosis of CVD, the implementation of sPLA2-llA is a good starting point for the diagnosis of diseases related to inflammation.5 Not only can sPLA2-llA be utilized in the diagnosis of CVD, but also in the prevention of secondary coronary events.13
REFERENCES
- World Health Organization. Cardiovascular Diseases. [Online] World Health Organization, May 17, 2017. [Cited: August 21, 2018.] https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- WebMD. What is Inflammation? [Online] WebMD. [Cited: January 22, 2019.] https://www.webmd.com/arthritis/about-inflammation#1
- Inflammation and Heart Disease. [Online] Heart.org. [Cited: January 22, 2019.] https://www.heart.org/en/health-topics/consumer-healthcare/what-is-cardiovascular-disease/inflammation-and-heart-disease
- World Health Organization (2018) The top 10 causes of death, http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (Accessed: June 22, 2018).
- Liu Michael. The need for Group IIA secretory phospholipase A2 (sPLA2 -IIA) inhibitors in inflammatory ocular disease treatment. Medical and Dental Research. 2018; Vol. 1.
- Kei Yamamoto. Secreted phospholipase A2, lipoprotein hydrolysis, and atherosclerosis: integration with lipidomics. Analytical and Bioanalytical Chemistry. 2011; Vol. 400.
- Mallat Z, Steg G, Benessiano J. Circulatory secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes. Journal of the American College of Cardiology. 2005; Vol. 46.
- Wilkinson A. Lp-PLA2 (PLAC) Test, Available at: https://tdlpathology.com/media/4932/TAP2268B_PLAC_InformationSheet_V5.pdf. (Accessed: July 3, 2018.)
- Mallat Z, Lambeau G, Tedgui A. Lipoprotein associated and secreted phopholipases A2 in CD. Circulation. 2010; Vol. 122.
- O’Donoghue M, Mallat Z, Morrow D. Prognostic Utility of Secretory Phospholipase A2 in Patients with Stable Coronary Artery Disease. Clinical Chemistry; 2011, Vol. 57.
- Benessiano Joelle. Circulating secreted phospholipase A2 activity: an early prognostic marker in unselected patients presenting toteh emergency department with suspected acute coronary syndrome. Science Direct. 2010; Vol. 2.
- Cook N, Paynter N, Manson J. Clinical utility of lipoprotein associated phospholipase A2 for cardiovascular disease prediction in multi-ethnic cohort of women. NCBI. 2012; Vol. 58.
- Koenig W. Association between type II secretory phospholipase A2 plasma concentrations and activity and cardiovascular events in patients with CAD. European Heart Journal. 2009; Vol. 30.
- Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006; 26: 1586–1593.
- Liu Ping-Yen. Prognostic value and the changes of plasma levels of secretory type II phospholipase A2 in patients with coronary artery disease undergoing percutaneous coronary intervention. Eurpoean Heart Journal. 2003; Vol. 24.
- NHS. Overview Sepsis. NHS. [Online] NHS. [Cited: December 3, 2018.] https://www.nhs.uk/conditions/sepsis/
- Tan Leong Toh, Goh Yew Yip. The role of group llA secretory phospholipase A2 (sPLA2-llA) as a biomarker for the diagnosis of sepsis and bacterial infection in adults - A systematic review. PLOS ONE, 2017.
- Mearelli F. Heterogeneous models for an early discrimination between sepsis and non-infective SIRS in medical ward patients: a pilot study. NCBI. 2014: Vol. 9.
- Truex P, Trias J. Methods for detection and measurement of secretory phospholipase A2 levels (sPLA2) in biological fluids. Google Patents. [Online] 2007. [Cited: May 1, 2019.]
- Lamon B, Hajjar P. Inflammation at the Molecular Interface of Atherogenesis: An Anthropological Journey. The American Journal of Pathology, 2008.
- Venos E, de Koning L. Endocrine markers of diabetes and cardiovascular disease risk. Alberta. Endocrine Biomarkers, 2017.