Non-small cell lung cancer (NSCLC) accounts for more than 80 percent of cases of lung cancer, which is a leading cause of cancer death in the United States, with a five-year survival rate of less than 18 percent. Recent developments in targeted therapeutics and immunotherapy and, importantly, progress in identification of biomarkers in NSCLC are rapidly changing clinical practice and bringing hope to patients with this devastating disease.
Noteworthy as a sign of progress are the multiple updates from the National Comprehensive Cancer Network (NCCN) guidelines that were issued in 2017 alone.1 These recommendations highlight four newly approved therapeutics, paralleled by three new biomarkers described within the past two years. The recommendations are included in both the NCCN guidelines and those issued jointly by the College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP).2
The late 2017 FDA approval of two biomarker assays with therapeutic indications is a further example of the strides being made in NSCLC testing. In this context, it is important to note that the key to improving patient survival is access—that is, getting timely results for the appropriate biomarkers is the necessary first step to receiving targeted therapy. More so than with many other diseases, the clinical laboratory can truly make an impact on the outcome for a patient with NSCLC. A clear understanding of the recommended biomarkers, their role in targeted therapy and immunotherapy, and testing methodologies available lays the groundwork for aligning the laboratory’s resources and processes to make sure patients receive the needed test results within the requisite turnaround time to guide life-changing clinical decisions.
Biomarkers of NSCLC
Biomarkers can guide clinical decision making in two ways. Predictive biomarkers, such as EGFR, are indicative of therapeutic efficacy and are used to identify patients who are likely to benefit from a specific therapy. The use of predictive biomarkers to select patients for targeted therapy is not only beneficial for the patients who receive the therapy; it is also valuable in helping avoid unnecessary treatment with associated side effects and costs for those who cannot benefit.
Prognostic biomarkers, on the other hand, are markers of patient survival independent of treatment received, signaling innate tumor aggressiveness and likelihood of disease progression or recurrence. One example of a prognostic biomarker in NSCLC is KRAS, where mutations are associated with poor survival and for which no targeted therapy is available today.
NCCN and CAP/IASLC/AMP guidelines recommend biomarker testing at diagnosis of NSCLC and at disease progression. Following is an overview of the predictive biomarkers recommended in the 2018 guidelines:
EGFR mutations include a number of mutations in the epidermal growth factor receptor (EGFR) gene. The most commonly found mutations are deletions in exon 19 and the L858R mutation in exon 21, both of which result in activation of the tyrosine kinase domain and are sensitive to small molecular tyrosine kinase inhibitors (TKIs) such as erloginib, gefitinib, and aftinib. These mutations are referred to as sensitizing EGFR mutations. After nine to 13 months of EGFR TKI therapy, however, most patients become resistant when an acquired mutation, EGFR T790M, may appear. NCCN guidelines specify T790M testing with disease progression to guide subsequent therapy.
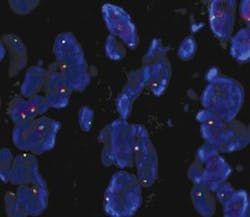
ALK gene rearrangements (Figure 1) are found in two percent to seven percent of patients with NSCLC. The anaplastic lymphoma kinase (ALK) fusion protein plays a key role in controlling cell proliferation, and gene rearrangement results in dysfunction and inappropriate signaling through the ALK kinase domain. Patients with ALK gene rearrangements have clinical characteristics including adenocarcinoma histology, never/light smokers, and younger age of onset. Confirmation of ALK rearrangements is a prerequisite to treatment with crizotinib, recommended by NCCN in 2017 as first-line treatment for ALK-positive metastatic NSCLC. NCCN guidelines recommend testing for ALK rearrangements in patients with nonsquamous NSCLC.
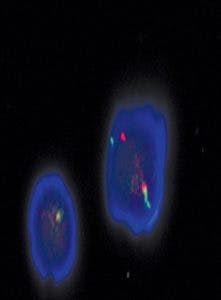
ROS1 gene rearrangements (Figure 2), which result from fusion of the ROS proto-oncogene 1 (ROS1) with partner genes such as CD74, SLC34A2, CCDC6, and FIFG, occur in one percent to two percent of patients with NSCLC, more frequently in younger women with adenocarcinoma who are never smokers, and in those negative for EGFR mutations and ALK gene arrangements. The presence of a ROS1 rearrangement is associated with responsiveness to oral TKIs such as crizotinib. NCCN began including ROS1 in the NSCLC testing algorithm in 2017. The CAP/IASLC/AMP 2017 guideline recommends ROS1 testing on all lung adenocarcinoma patients.
BRAF (p.V600E) mutation, a mutation in amino acid position 600 (p.V600E) of the B-Raf proto-oncogene, is found in five percent of NSCLC. It is associated with responsiveness to combined therapy with dabrafenib, an oral inhibitor of BRAF, and with trametinib, which inhibits the mitogen-activated protein kinase enzymes MEK1 and MEK2. There are other BRAF mutations associated with NSCLC—p.V600E represents 50 percent of BRAF mutations in NSCLC—but the impact of these other mutations on therapy selection is not well understood. Testing for BRAF mutations is recommended for patients with nonsquamous NSCLC and may be considered in patients with squamous cell NSCLC.
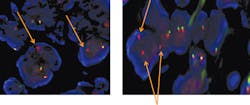
PD-L1 (programmed death ligand 1) (Figure 3), is a protein expressed by tumor cells to inhibit T-cell mediated cell death by binding to PD-1, a receptor expressed on activated cytotoxic T-cells. This is one of the evasion mechanisms employed by tumor cells to weaken the immune system’s response to cancer. Immunotherapeutic agents such as nivolumab, pembrolizumab, durvalumab, and atezolizumab improve antitumor effects of T-cells by blocking PD-L1 and PD-1 interaction. NCCN guidelines recommend testing for PD-L1 expression before first-line treatment in patients with metastatic NSCLC when results from EGFR and ALK are negative.
From guidelines to practice
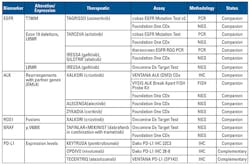
Table 1 summarizes commercially available tests for the predictive biomarkers included in the 2018 NCCN and CAP/IASLC/AMP guidelines. These have received FDA clearance as in vitro diagnostics (IVD). In addition, laboratory-developed tests (LDTs), which are designed, manufactured, and used within a single lab, are available through labs offering testing services.
In the U.S., a predictive biomarker assay can be designated a companion diagnostic or a complementary diagnostic by the FDA at the time of approval. The FDA defines a companion diagnostic as one that “provides information that is essential for the safe and effective use of a corresponding drug or biological product.” A positive result is required for the patient to receive the therapeutic. A relatively new concept, a complementary diagnostic, “aids in the benefit-risk decision-making about the use of the therapeutic product, where the difference in benefit-risk is clinically meaningful.” Thus, a complementary diagnostic is also predicated upon identifying patients most likely to benefit from the therapeutic but does not preclude the clinician from prescribing the drug to patients who test negative. This is because therapeutic benefit has been demonstrated in patients regardless of biomarker status. This gives physicians more discretion in deciding which patients are likely to benefit from a therapeutic.
Developing a testing strategy
The effectiveness of NSCLC biomarker testing is driven by multiple and often interrelated factors including patient care needs, lab resources and infrastructure, and quality practices from specimen collection through reporting of results.3 When choosing a test methodology, timeliness of information for clinical decision making is an important consideration. This is especially true in the setting of NSCLC, a rapidly progressing disease for which targeted therapy for appropriate patients not only improves survival but also reduces side effects often associated with traditional therapy. For example, in ALK testing, an automated immunohistochemistry (IHC) platform, compared with a manual fluorescence in situ hybridization (FISH) test, can significantly reduce turnaround time and make same-day result reporting possible. The choice of testing methodology is also driven by lab resources. The choice between IHC and FISH might come down to whether a cytogenetics lab, or just a histology lab, is part of the overall lab structure.
The problem with innately small lung tissue samples, often with sparse cancer cell content, and the need to conserve tissue for various tests as well as participation in clinical trials, point to the importance of offering tests that provide useful clinical information. For example, both the CAP/IASLC/AMP and NCCN guidelines recommend against total EFGR expression by IHC testing because the predictive effects of specific drug-sensitive mutations are well defined. Along these lines, test methodologies that require smaller amounts of samples are preferred. The 2017 CAP/IASLC/AMP guideline includes a statement of expert consensus opinion that labs should use, or have available through an external reference lab, assays that are able to detect molecular alterations in specimens with as little as 20 percent cancer cells. For EGFR T790M testing, the test must be able to detect the mutation in as few as five percent of cells. In this context, testing DNA in plasma samples offers a welcome change, since it is less invasive and may enable more patients to be tested. However, plasma-based testing can be influenced by variables such as differences in tumor biology and the amount of DNA released from the tumor cells. NCCN recommends reflexing to tissue-based testing if a plasma EGFR test is negative for the T790M mutation.
Indeed, specimen quality, as defined by sufficient tumor quantity to allow for standard histologic evaluations and biomarker testing, is the first step in identifying patients for targeted therapy. Currently, only 62 percent of eligible patients are tested at disease progression, while 82 percent of patients would agree to undergo biopsy if it would help to provide access to additional therapy options.
Last but not least, key to successful biomarker testing is a multidisciplinary approach bringing in all of the stakeholders, including the patient, from patient identification through test result reporting. The goal should be clear communication and understanding of each stakeholder’s role in this journey. Beyond implementation of a quality biomarker testing program for NSCLC, the clinical laboratory professional holds a key role in communicating with clinicians and keeping them aware of new developments and how to work with the lab to leverage these developments for their patients’ benefit.
REFERENCES
- NCCN Guidelines: Non-Small Cell Lung Cancer. Version 2.2018, December 2017. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology [published online January 22, 2018]. J Thorac Oncol. 2018;13(3): 323-358.
- Diagnostic testing in metastatic non-small cell lung cancer. Webinar,
October 2017. https://www.youtube.com/watch?v=E4asuE5Fsfs.
Virginia Thurston, PhD, FACMG, serves as Director of Cytogenetics for Carolinas Pathology Group and Carolinas HealthCare System, an integrated health network with more than 40 hospitals. She oversees clinical cytogenetic and molecular cytogenetic testing for inherited diseases, hematologic malignancies, and solid tumors.
John W. Longshore, PhD, FACMG, serves as Director of Molecular Pathology for Carolinas Pathology Group and Carolinas HealthCare System. He oversees molecular testing for inherited disease, virology, microbiology, hematologic malignancy, and solid tumor analysis.