Sepsis is a serious medical condition characterized by potentially fatal whole-body inflammation resulting from infection. It is a common condition with high mortality rates in the United States and around the world. The burden it imposes on the healthcare system is large. In the U.S., sepsis is the 11th most common cause of death and accounts for one in every 10 ICU admissions each year. Sepsis has been highlighted as a major issue in the clinical community, and faster diagnosis and treatment of sepsis has been a major focus of emergency medicine over the last decade. There is intense pressure to improve management of sepsis, from earlier identification and administration of antimicrobial therapy to monitoring and de-escalation of therapy.
Much of the emphasis and improvement in diagnosis and treatment is the byproduct of efforts by the Surviving Sepsis Campaign, initiated in 2002, whose guidelines have raised awareness of how to identify and manage the condition. Many hospitals now implement specialty workgroups and taskforces for early identification and management of sepsis. A key component of the protocols being implemented in these hospitals is the testing of inflammatory markers that can help identify sepsis at an earlier stage to provide guidance for antimicrobial therapy, provide rule-out information, and improve patient outcomes. Since time-to-diagnosis and immediacy of intervention is essential to the successful management of sepsis, the condition is an excellent candidate for point-of-care testing (POCT).
Expanding use of POCT for sepsis
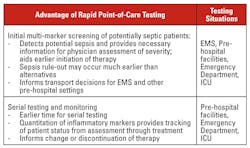
Rapid point-of-care testing has been available for many years. However, the use of rapid point-of-care diagnostic testing as it relates to sepsis today remains limited to the routine testing of lactate levels, a useful but nonspecific marker of sepsis. Moreover, many of the available testing systems have been difficult to use and have not delivered the quality of results that are required for clinical support in critical patient situations, resulting in a degree of physician resistance to adopting rapid testing practices. Now, however, given technological advances in sensors and detection systems, we are beginning to see POCT products that can deliver insightful clinical information, are easy to use, and can be broadly deployed. The advantages of point-of-care testing systems are summarized in Table 1.
Sepsis testing and diagnosis should be viewed as a two-phase process:
- Phase 1: immediate patient testing and assessment focused on emergency intervention and immediate entry into sepsis treatment protocols;
- Phase 2: specific identification of the source of infection and related adjustments in clinical intervention.
Current diagnostic testing practices are focused primarily on Phase 2, identification of the pathogen that is the primary source of infection. The gold standard for this testing remains blood culture, with a growing use of molecular diagnostic tools. The turnaround time for blood culture results can exceed 24 hours, and molecular testing often takes several hours or more to deliver test results. These tests are focused on pathogen identification, and quantification of infection load is usually unavailable.
Phase 1 is the critical first step of patient assessment and diagnosis. Testing in this immediate emergency phase could be improved through the use of rapid measurement of biomarkers that rise when sepsis is present. In addition to the already measured lactate, two such biomarkers are C-reactive protein (CRP) and procalcitonin (PCT). To date, there has been very limited availability of rapid tests for either of these two markers. Most testing is done in the central laboratory, with several-hours-to-a-day turnaround time (TAT), and often requiring multiple testing platforms. From a practical standpoint, diagnostic information needs to be available in less than 30 minutes to be useful in the initial phase of patient assessment and treatment. Beyond 30 minutes, decisions will have been made and patients will have been redirected.
A new alternative emerges
A panel that tests lactate, CRP, and PCT has recently been developed in a fully automated, quantitative, rapid point-of-care diagnostic system, wherein all three tests can be run simultaneously from a single patient sample (whole blood or plasma) with a TAT of 10 minutes. This testing approach reduces cost, streamlines testing, increases workflow efficiency, and rapidly delivers useful clinical information.
The technological challenge for POCT systems is to deliver simultaneous detection of both protein markers (CRP and PCT) and enzymes (LAC) so that one system can be used for quantification of the three relevant biomarkers. Precise quantification of each of these markers can add important information for the initial patient evaluation and can also support ongoing patient monitoring. To add usefulness and efficiency of testing and patient triage, the rapid testing system should be deployable inside or outside of a hospital setting, including potential for use by paramedics and first responders, urgent care clinics, and other decentralized care centers. Outside of the hospital, rapidly available test information and wireless communication protocols aid immediate sharing of data and decisions about patient care, including the need to transport patients and appropriate patient destination. The characteristics of the sepsis panel discussed above are summarized in Table 2.
New molecular diagnostic testing solutions have greatly improved the usability and timeliness of molecular testing. However, molecular tests look for the presence or absence of a particular pathogen rather than assessing the infection load of the patient, and thus cannot provide the advantage offered by using biomarkers of infection in the rapid test format. For molecular testing to become a useful addition to rapid emergency diagnostic testing, sample-to-result TAT needs to be less than 30 minutes, and ease of use needs to be improved to the point where testing systems can be run by multiple skill-level operators.
Further advantages of POCT devices
Speed to intervention is critical to improving sepsis patient outcomes. Rapid test systems can be used to deliver results in as little as 10 minutes at the first point of patient interaction and have the potential to be an essential management tool to highlight and focus the physician and hospital on high-risk patients for immediate attention and intervention. Rapid test systems can offer numerous advantages such as rapid quantitative testing, mobility, ease of use, and connectivity.
In addition to the rapid availability of diagnostic information, POCT devices can also significantly impact the cost and efficiency of care. The National Academy of Medicine estimates that $750 billion annually is spent on unnecessary medical services, inefficient delivery, excessive administrative costs, and prevention failures.1 Testing patients at the point of first contact can help reduce a significant amount of these excess costs. In addition to reducing the time to diagnosis in hospital environments, portable rapid testing technology can also be used in situations outside the hospital to help determine if patients need to be transported to a hospital for treatment, if they need to go to a specific healthcare facility, or if they can be treated in place.
Rapid test systems will be used in settings such as skilled nursing facilities, elderly care centers, convalescent centers, and urgent care clinics, to help diagnose or monitor patients. Reducing the time to diagnosis and treating patients in place can not only increase patient satisfaction and improve outcomes, but could also significantly lower costs. POC systems will be most helpful in those situations where diagnostic information is necessary to help inform and direct clinical intervention, such as myocardial infarction, stroke, brain injury—or sepsis.
REFERENCE
- Hagland M. IOM report, The path to continuously learning healthcare in America,. Healthcare informatics : the business magazine for information and communication systems. 2012;29(9):30-33. Epub 2012/12/01.
David Ludvigson is President and CEO of Emeryville, California-based Nanomix, Inc., a company implementing mobile, rapid diagnostic testing for critical disease states using a proprietary handheld platform.