Optimizing and standardizing testing to address the threat of chronic kidney disease
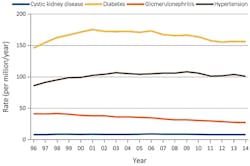
Understanding chronic kidney disease (CKD) is important not only because of its personal and economic toll, but also because of its relationship to diabetes. Long-term chronic diabetes is a major cause of nephropathy, which leads to CKD, and in fact diabetes is the leading cause of CKD, creating about 50 percent of all cases (Figure 1).1,2 Diabetes is a major cause of nephropathy, which leads to chronic kidney disease. Diabetes affects 415 million people worldwide, and that number is expected to reach 642 million by the year 2040.3
Chronic hypertension is the second leading cause of CKD, accounting for approximately one-third of CKD cases.1 Thus major efforts at reducing CKD are focused on treating the underlying diabetes and hypertension at the root of so many cases.
CKD places a great burden on the healthcare system at large. While an estimated 30 million U.S. adults suffer from CKD,1 end-stage renal disease (ESRD), the most severe of the five stages of CKD defined by the National Kidney Foundation, affects up to two million people.4 Growing at a rate of five percent to seven percent each year, ESRD is a designation reserved for patients on dialysis or awaiting kidney transplantation. Dialysis is taxing for patients, and only 35 percent of those on dialysis live beyond five years of treatment.4 From an economic perspective, in the U.S. the annual cost of hemodialysis is $42 billion, and the cost of kidney transplantation is estimated at $55,000 for initial surgery and a year of post-surgery care.4
Defining CKD
In 2012, the Kidney Disease: Improving Global Outcomes organization (KDIGO) characterized CKD as abnormalities in the kidney’s structure or function, lasting for three or more months and having serious implications for patient health. KDIGO identified criteria for CKD, including kidney damage markers via imaging, altered renal function blood markers, and decreased glomerular filtration rate (GFR).5
Among the significant additions made by KDIGO were the inclusion of urine albumin, also called micro-albumin, as a key factor in assessing CKD, whereas, previously, the disease had been predominantly diagnosed using creatinine and GFR. The cause of the CKD was also considered in classification of the patient. Thus, the organization recommended classifying CKD based on the CGA model, which takes into account cause, GFR category, and albuminuria category (Table 1).5 The patient’s classification then determines practice guidelines for follow-up protocols.
Biomarkers: the importance of standardization
Today, there are three primary measured biomarkers used in CKD diagnosis and management: creatinine, urine albumin (UAlb), and cystatin C. The calculation of GFR is also needed. Effective testing—and, thus, effective disease management—is reliant on test standardization, the ability of different test methods to provide the same test result. A lack of standardization in testing potentially causes variances in disease diagnosis, patient risk categorization, and treatment protocols.
Creatinine. Creatinine has become a success story in terms of standardization. The original work of the National Kidney Disease Education Program (NKDEP) Lab Working Group focused on creatinine standardization by specifying reference methods, traceability, and performance requirements.6 The success of this program can be seen in the very close agreement of all test method peer group results on surveys such as the College of American Pathologists (CAP) Proficiency Testing program.
The two primary approaches to measuring creatinine are the Jaffe method and the enzymatic method. Each has its advocates in terms of convenience, cost, and effect of interferences, but both give the same test result, because of the work done on
standardization.
eGFR. eGFR is a measurement of kidney function based on an equation that includes not only creatinine levels, but also age, gender, and race. This offers a better picture than creatinine testing alone can provide. The two most widely applied equations for estimating GFR in adults are the MDRD and the CKD-EPI equations. Because all creatinine measurements are now well-standardized, differences in adult GFR results between labs are often due to errors in programming the equations.
The MDRD equation has been used more traditionally; however, recent guidelines recommend the newer CKD-EPI equation over the MDRD, and it is becoming more common.
A different GFR calculation, the Schwartz equation, is used for pediatric patients. And pharmacists rely on the Cockroft-Gault equation for GFR, as specified in drug-dosing regimens where the drug dose is dependent on assessment of kidney function. Thus, labs should be careful to communicate with pharmacy professionals about GFR calculations, and provide correlation information regarding equation calculations, standardization, and reporting methods that may affect drug dosing.
Urine albumin. Urine albumin testing, also known as microalbumin and reported as an albumin-creatinine ratio, is the second assay now recommended by clinical practice guidelines.5 Albumin is a protein that is not found in urine when the kidneys are operating normally. When the kidney’s glomerulus begins to deteriorate, however, it allows the protein to pass through, and, when detected in urine, is an indicator that there is a degree of kidney dysfunction. The greater the concentration of urine albumin, the greater the degree of kidney damage. Urine albumin tests are not nearly as standardized as creatinine, so there is some variation in results among test methods.7 However, efforts are underway to address this test variation relative to an established reference method. While some manufacturers offer assays that are well standardized, assays from other manufacturers may produce different results. Awareness of lack of standardization among test methods is important, as urine albumin test results may affect clinical practice.
Cystatin C. Cystatin C is another protein that, when detected in high levels in the blood, indicates kidney disease. It is often used in place of or along with creatinine levels in calculating GFR.8 The KDIGO-recommended guidelines for cystatin C testing include measuring the protein in adults with GFR between 45 and 59 to confirm CKD in the absence of other markers for kidney damage. Additionally, when measuring cystatin C, clinicians should use a GFR estimating equation to calculate the GFR from serum cystatin C, instead of depending upon serum cystatin C concentration alone.5,8
Cystatin C results can vary among methods, depending upon the standardization and traceability of the test method. The development of an international reference material now allows manufacturers to standardize against a common standard, and some have already done so. KDIGO further recommends that clinical laboratories measuring cystatin C use assays calibrated to the international standard reference material.5
On the rise: serum albumin
Serum albumin testing has become a part of routine care for ESRD patients, particularly those receiving dialysis. As a general indicator of a patient’s overall health, it not only provides information about fluid status, nephrosis, and liver function, but is also recommended by the NKF KDOQI as a guide for monitoring proper nutritional status in patients on maintenance renal dialysis.9 Because serum albumin has caught the attention of government agencies in evaluating the well-being of patients on dialysis, there is high interest in ensuring test standardization to ensure accurate reflection of dialysis center performance and patient care.
CKD is a worldwide threat that is both under-diagnosed and under-treated. Moreover, it is a growing threat, due to anticipated increases of diabetes and hypertension in the aging population. At the center of the fight against the progression of CKD are clinical laboratories, which play an important role in addressing CKD, educating clinicians and pharmacy professionals, and developing new markers and equations to bring better diagnostic and treatment options. The growing use of urine albumin, cystatin C, and serum albumin bring new promise to the area of CKD management, but better test standardization practices are needed to continue to advance progress.
REFERENCES
- Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet, 2017. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
- United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Disease, Bethesda, MD, 2016. https://www.usrds.org/adr.aspx.
- Diabetes: facts and figures. International Diabetes Federation. http://www.idf.org/about-diabetes/facts-figures.
- U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013.
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, Kidney International Supplements, Vol 3, January 2013.
- Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5-18.
- Bachmann LM, Nillson G, Bruns DE, et al. State of the art for measurement of urine albumin: comparison of routine measurement procedures to isotope dilution tandem mass spectrometry. Clin Chem. 2014;60(3):471–480.
- Grubb A, Horio M, Hansson LO, et al. Generation of a new cystatin c–based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem. 2014;60(7):974–986.
- Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. K-DOQI National Kidney Foundation. Am J Kidney Dis. 2000;35(6 Suppl2):S1-140.
Jack Zakowski, PhD, FACB, serves as Director of Scientific Affairs and Professional Relations for Beckman Coulter Diagnostics.
About the Author

Jack Zakowski, PhD, FAACC
is Principal of IVD Consulting, LLC and chaired the development of CLSI C56A, Hemolysis, Icterus, and Lipemia/Turbidity Indices as Indicators of Interference in Clinical Laboratory Analysis.He has served in governance positions with AACC, is Past President of CLSI, and is currently Chair of ISO TC212 Clinical Laboratory Testing and in vitro diagnostic Test Systems.