Advances in personalized medicine: Prognostic testing in patient management
Laboratory tests inform most clinical decisions and are essential for optimal patient management. Most existing assays are diagnostic, supporting determination of the presence or absence of a disease or condition at the time of testing. As we move into the era of personalized (or precision) medicine, testing at the individual level for disease progression or response will become increasingly important, enabling tailored management to improve individual outcomes. Foretelling disease course or risk-assessment necessitates tests or scores validated for predictive performance, including assays with specific prognostic data and claims.
Prognostic testing indicates risk of future events, such as disease progression or recurrence and is typically based on outcome data over a defined period (e.g., five years).1 Prognostic testing is sometimes divided into “prognostic” to predict the natural course of disease and thus who might benefit from treatment, and “predictive” to indicate outcomes with treatment. Frequently, the terms are used interchangeably. To better guide patients’ treatment choices and drive improved outcomes, prognostic testing is increasingly being integrated into the management of a small but expanding number of diseases, including breast cancer, cardiovascular, and liver disease.
Prognostic testing in breast cancer
One in eight. The number of women who will receive a diagnosis of breast cancer in the United States. While recent and encouraging data shows survival rates are improving,2 breast cancer remains a highly heterogeneous disease involving multiple treatment decisions from the moment of diagnosis. Early breast cancer is increasingly treatable but requires determinations such as if chemotherapy is likely to be beneficial in the adjuvant (post-surgical) setting. Is there a difference in individual outcomes at five or ten years if electing to forgo chemotherapy (and its associated risks) with early disease or is treatment always beneficial?
In the case of early breast cancer, genetic information from the tumor itself can now be added to traditional risk factors such as tumor stage, size, and hormone receptor status. Complex molecular tests utilizing gene expression profiling can analyze multiple ribonucleic acid (RNA) transcripts in the individual tumor and generate a mathematical risk score (typically grouped as high, intermediate, or low) that incorporates longitudinal outcomes study data to predict risk or benefit of treatment.3,4
Tumor profiling following a breast cancer diagnosis contrasts with other molecular risk testing such as BRCA 1 and 2 (breast cancer gene 1 and breast cancer gene 2) that is associated with risk of cancer occurrence based on mutations in the individual’s genomic DNA. Following surgical removal, the patient’s tumor is analyzed for gene expression specific to that individual’s cancer. Differing tumor-specific molecular profiles have been associated with risk of recurrence, the benefit of chemotherapy, or the extension of adjuvant endocrine therapy. For example, a score can indicate as little as one percent benefit if electing for chemotherapy in the adjuvant setting, supporting a decision to forgo treatment and avoid the concomitant risks. Alternatively, a high score can predict improved outcomes with treatment. While only one component in the complex management of breast cancer, an expanding number of prognostic molecular tests are increasingly recommended in guidelines,4,5 integrated into management, and are gaining reimbursement from insurers.
Prognostic testing in cancer incorporates multiple approaches
Other markers employed prognostically in breast cancer include estrogen receptor (ER) and HER2 positivity used to predict benefit of antiestrogen and anti-HER2 therapies. Ki67 immunohistochemistry, a marker of breast cancer proliferation, is finding prognostic usefulness, including a recent recommendation for use in ER-positive, HER-2 negative early breast cancer with limited or no node involvement.6
On-going research continues to investigate promising new prognostic and predictive biomarkers for breast and other cancers. Current research includes circulating tumor DNA (ctDNA), micro RNAs, and circulating tumor cells.7 One promising biomarker is the mutational status of the estrogen receptor (ESR1) for predicting the emergence of resistance to aromatase inhibitors in HR-positive advanced breast cancer.8 Other investigations include biomarkers for predicting response to radiotherapy treatment and specific forms of chemotherapy. Prognostic testing in the field of oncology is a leading example for the potential of personalized medicine to customize clinical management and enhance individual outcomes.
Differentiating risk assessment tools from prognostic assays
It is important to distinguish tests that are diagnostic but can be incorporated into a risk-assessment tool versus an assay with a specific claim or authorization such as prognostic gene expression profiling. While molecular assays comprise most current tests with specific prognostic claims and applications, a small but growing number of blood-based tests, including immunoassays, are anticipated/have achieved FDA-authorization specific to a prognostic use. However, diagnostic assays can be incorporated into scoring tools for risk prediction, for example to estimate probabilities for cardiovascular disease (CVD).9 CVD risk scores typically incorporate clinical findings and other information such as history, age, and gender along with diagnostic assay results.
Prognostic testing in CVD
Cardiovascular disease (CVD) risk scores are increasingly being utilized in both acute and nonacute applications. In the outpatient setting, assessment tools such as the American College of Cardiology/ American Heart Association ASCVD risk estimator or the Framingham CVD risk score integrate diagnostic test results such as total cholesterol and HDL into the multifactorial calculator. The ACC/AHA and Framingham risk scores predict the likelihood of a CVD event or coronary heart disease over a 10-year period, so they can be utilized as a prevention strategy to mitigate disease through proper interventions such as therapy or lifestyle modifications. While it is well-accepted that high cholesterol or low HDL portends risk, it is the aggregate risk-assessment score that is prognostic versus the diagnostic assay values.
Related CVD risk scores can also be highly useful in the acute setting.10 Most chest pain patients with signs and symptoms of a non-ST elevated myocardial infarction (NSTEMI) are not experiencing a myocardial infarction, but it must be safely ruled out prior to discharge or assessment for alternate etiologies. While serial changes in high-sensitivity troponin are utilized in the diagnostic pathway, risk scores such as HEART or TIMI that include troponin are often incorporated in the assessment and can be especially useful for the low-risk rule-out. While used to aid risk-assessment in a scoring tool, the troponin assay remains a diagnostic test.
Intriguingly, however, high-sensitivity cardiac troponin assays are being investigated as a direct marker of risk, and in Europe some have achieved a specific prognostic claim utilizing values well below the diagnostic threshold of the 99th percentile. The use of high-sensitivity cardiac troponin assays has been recently recommended for CVD risk prognostication in the general population.11,12 While currently no high-sensitivity assays in the United States have been cleared by the FDA with a prognostic claim, it is an area of active research.
Prognostic testing in liver disease: A growing urgency
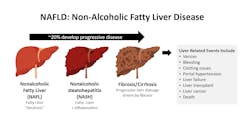
Greater than 100 million people in the United States suffer (most unknowingly) from chronic liver disease (CLD), the leading cause being nonalcoholic fatty liver disease (NAFLD) affecting up to an estimated 30% of the U.S. population.13 NAFLD encompasses a spectrum of liver pathology, from simple steatosis (fatty liver) to higher-risk disease associated with advancing fibrosis, cirrhosis, liver failure, or hepatocellular carcinoma. (See Figure 1.) Comorbidities such as obesity, diabetes, and the metabolic syndrome increase risk for NAFLD and disease progression and include “lean NAFLD” occurring in non-obese individuals.13,14 About 25% of people with fatty liver will develop nonalcoholic steatohepatitis (NASH), an inflammatory form more likely to advance to cirrhosis and liver failure.
Factors such as genetics, the gut microbiome, diet, and lifestyle are contributing variables associated with disease progression.15,16 Identifying progressors from non-progressors is challenging due to the frequently silent nature of advancing damage and the sheer numbers of those with fatty liver. Moreover, a significant majority of patients with steatosis and/or elevated liver enzymes do not develop progressive disease, and many with high-risk disease may have normal liver enzyme levels.17
The need for earlier identification
Of paramount concern is the high frequency of those diagnosed only after cirrhotic decompensation where few options besides transplant exist, and hospitalization for complications are frequent.18 The need for an effective risk-stratification strategy in NAFLD is critical, as earlier identification and intervention can limit or reverse disease, even some cases of compensated cirrhosis. Moreover, promising pharmacologic treatments for NAFLD and NASH are in late-stage development and may soon become available.16 But what is a good strategy to optimize outpatient management and prevent unnecessary referrals, hospitalizations, and deaths associated with cirrhosis? For that, it is important to understand the underlying pathology.
Liver fibrosis and disease progression
Evidence clearly shows it is not inflammation but rather the active formation of scar tissue—fibrosis—that is the strongest predictor of disease advancement.19-21 Fibrosis is more likely to occur with inflammation (NASH), although may occur in its apparent absence. Fibrosis is a natural part of the wound healing process, with damage (fibrogenesis) existing in a balance with tissue repair mechanisms (fibrolysis). With chronic exacerbation, that balance is altered, damage overwhelms repair and healthy liver tissue is increasingly injured and scarred.
Evaluation for evidence of active fibrosis is a proven means of risk-assessment; techniques include biopsy and noninvasive testing (NIT) using imaging or biomarkers.22-24 Biomarker testing methods include measurement for direct markers that indicate active fibrosis or indirect markers related to inflammation or damage.
Liver fibrosis and biopsy
Histopathology (liver biopsy) has been the historical standard to indicate inflammation (activity or grade) and fibrosis (staging) for disease severity. A scoring system (commonly stages 0–4 with 4 indicating cirrhosis) is typically reported. While biopsy is a diagnostic technique, data show it is also predictive of disease progression, with higher stages associated with elevated risk.19-21 However, biopsy has significant limitations including invasiveness and risk, sampling error, and inter-observer variability.25 To better address the unmet clinical need, including high-volume testing, NITs using imaging for elastography or for direct biomarkers of fibrosis have recently been incorporated into U.S. guidelines for risk-assessment in NAFLD.14,15
NITs for liver fibrosis and risk-prediction
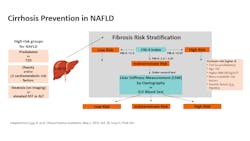
As fibrosis often proceeds slowly (though some are fast progressors), identification of active fibrosis indicates elevated risk. Successful intervention is linked with disease reversal and improved outcomes but must be timely (prior to decompensated cirrhosis).26-28 As higher-risk patients (including those with obesity and/or diabetes) are most likely to be seen initially in primary care or endocrinology settings, testing recommendations target these clinicians. Algorithms in the United States designed for cirrhosis prevention include a simple blood-based NIT (FIB-4 index) for the initial screening and secondary NIT testing for additional risk-stratification and referral decisions.14,15 (See Figure 2.)NITs: Blood based vs. imaging
NITs for liver fibrosis fall into two general categories: blood-based biomarkers or imaging for “liver stiffness” (elastography).22-24 Imaging includes ultrasound/ultrasonic or MRI-based techniques that can assess for liver stiffness, as advancing fibrosis produces progressively less flexible tissue. Blood-based NITs include those using indirect markers (such as inflammatory markers or markers of liver dysfunction associated with fibrotic damage), or direct markers of fibrosis involved in fibrogenesis and/or fibrinolysis. Both imaging (liver elastography) and serum-based direct markers (using the enhanced liver fibrosis [ELF] blood test, which automatically measures three direct markers to produce a numeric risk score) are included as NIT options following an elevated FIB-4.14,15 ELF is the first blood-based test to receive De Novo authorization from the FDA as a prognostic assay in NASH, though others are in development.29 In the United States, the ELF Test is not for use in the diagnosis of NASH or for the staging of fibrosis. The ELF test is indicated as a prognostic marker in conjunction with other laboratory findings and clinical assessments in patients with advanced fibrosis (F3 or F4) due to NASH to assess the likelihood of progression to cirrhosis and liver-related clinical events.
Conclusion
A growing number of promising assays with specific prognostic utility across diverse disease states are anticipated in the coming years and include neurology, cardiac, liver disease, and cancer as active areas of investigation. Additionally, AI-assisted risk algorithms are showing promise across a range of morbidities. The increasing availability of risk-assessment tools and assays is expected to advance the promise of personalized medicine and reduce hospitalization and patient burden.
References
1. Mathes T, Pieper D. An algorithm for the classification of study designs to assess diagnostic, prognostic and predictive test accuracy in systematic reviews. Syst Rev. 2019;8(1):226. doi:10.1186/s13643-019-1131-4.
2. Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524-541. doi:10.3322/caac.21754.
3. Markopoulos C, Hyams DM, Gomez HL, et al. Multigene assays in early breast cancer: Insights from recent phase 3 studies. Eur J Surg Oncol. 2020;46(4 Pt A):656-666. doi:10.1016/j.ejso.2019.10.019.
4. Vieira AF, Schmitt F. An update on breast cancer multigene prognostic tests-emergent clinical biomarkers. Front Med (Lausanne). 2018;5:248. doi:10.3389/fmed.2018.00248.
5. Andre F, Ismaila N, Henry NL, Somerfield MR, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: ASCO Clinical Practice Guideline Update-Integration of Results From TAILORx. J Clin Oncol. 2019;1;37(22):1956-1964. doi:10.1200/JCO.19.00945.
6. Nielsen TO, Leung SCY, Rimm DL, Dodson A, et al. Assessment of Ki67 in breast cancer: Updated recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2021;1;113(7):808-819. doi:10.1093/jnci/djaa201.
7. Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. 2018;52(Pt 1):56-73. doi:10.1016/j.semcancer.2017.08.010.
8. Brett JO, Spring LM, Bardia A, Wander SA. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;15;23(1):85. doi:10.1186/s13058-021-01462-3.
9. Abidov A, Chehab O. Cardiovascular risk assessment models: Have we found the perfect solution yet? J Nucl Cardiol. 2020;27(6):2375-2385. doi:10.1007/s12350-019-01642-x.
10. Gulati M, Levy PD, Mukherjee D, Amsterdam E, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;30;144(22):e368-e454. doi:10.1161/CIR.0000000000001029.
11. Farmakis D, Mueller C, Apple FS. High-sensitivity cardiac troponin assays for cardiovascular risk stratification in the general population. Eur Heart J. 2020;1;41(41):4050-4056. doi:10.1093/eurheartj/ehaa083.
12. Clerico A, Zaninotto M, Passino C, Aspromonte N, et al. Evidence on clinical relevance of cardiovascular risk evaluation in the general population using cardio-specific biomarkers. Clin Chem Lab Med. 2020;21;59(1):79-90. doi:10.1515/cclm-2020-0310.
13. Younossi Z, Henry L. Non-alcoholic steatohepatitis: Global impact and clinical consequences. Eur Med J Hepatol. 2022;10(1):74-83. doi:10.33590/emjhepatol/22-00150.
14. Long MT, Noureddin M, Lim JK. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology. 2022;163(3):764-774.e1. doi:10.1053/j.gastro.2022.06.023.
15. Cusi K, Isaacs S, Barb D, Basu R, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28(5):528-562. doi:10.1016/j.eprac.2022.03.010.
16. Dufour JF, Anstee QM, Bugianesi E, Harrison S, et al. Current therapies and new developments in NASH. Gut. 2022;16;71(10):2123–34. doi:10.1136/gutjnl-2021-326874.
17. Ahmed Z, Ahmed U, Walayat S, Ren J, et al. Liver function tests in identifying patients with liver disease. Clin Exp Gastroenterol. 2018;23;11:301-307. doi:10.2147/CEG.S160537.
18. Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;1;399(10319):61-116. doi:10.1016/S0140-6736(21)01701-3.
19. Dulai PS, Singh S, Patel J, Soni M, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65(5):1557-1565. doi:10.1002/hep.29085.
20. Taylor RS, Taylor RJ, Bayliss S, Hagström H, et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158(6):1611-1625.e12. doi:10.1053/j.gastro.2020.01.043.
21. Hagström H, Nasr P, Ekstedt M, Hammar U, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265-1273. doi:10.1016/j.jhep.2017.07.027.
22. Patel PJ, Connoley D, Rhodes F, Srivastava A, Rosenberg W. A review of the clinical utility of the Enhanced Liver Fibrosis test in multiple aetiologies of chronic liver disease. Ann Clin Biochem. 2020;57(1):36-43. doi:10.1177/0004563219879962.
23. Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69(7):1343-1352. doi:10.1136/gutjnl-2018-317593.
24. Younossi ZM, Noureddin M, Bernstein D, Kwo P, et al. Role of Noninvasive Tests in Clinical Gastroenterology Practices to Identify Patients With Nonalcoholic Steatohepatitis at High Risk of Adverse Outcomes: Expert Panel Recommendations. Am J Gastroenterol. 2021;1;116(2):254-262. doi:10.14309/ajg.0000000000001054.
25. Filozof CM, Lackner C, Romero-Gómez M, et al. Best practices in liver biopsy histologic assessment for nonalcoholic steatohepatitis clinical trials: Expert opinion. GastroHep. 2022;2022:1-11. doi:10.1155/2022/3538103
26. Chalasani N, Abdelmalek MF, Loomba R, Kowdley KV, et al. Relationship between three commonly used non-invasive fibrosis biomarkers and improvement in fibrosis stage in patients with non-alcoholic steatohepatitis. Liver Int. 2019;39(5):924-932. doi:10.1111/liv.13974.
27. Tacke F, Weiskirchen R. Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)-related liver fibrosis: mechanisms, treatment and prevention. Ann Transl Med. 2021;9(8):729. doi:10.21037/atm-20-4354.
28. Reversing advanced fibrosis caused by NASH. AJMC. Published February 24, 2020. Accessed January 31, 2023. https://www.ajmc.com/view/reversing-advanced-fibrosis-caused-by-nash.
29. Device Classification Under Section 513(f)(2)(De Novo). Accessed January 31, 2023. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/denovo.cfm?id=DEN190056.
About the Author

Katherine Soreng, PhD
currently heads Global Clinical Education for Laboratory Diagnostics at Siemens Healthineers. She writes and educates on a variety of clinical diagnostic disease states, including Infectious Disease, Liver Fibrosis, Cardiology, and Neurology. Dr. Soreng completed her BS in Biology at the University of Washington in Seattle and her PhD in Immunology and Molecular Pathogenesis at Emory University in Atlanta, GA.