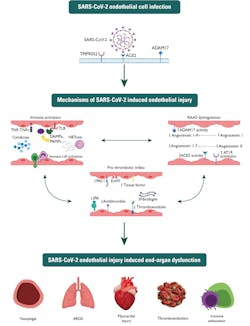
SARS-CoV-2 and vascular and multi-system dysfunction
COVID-19, the disease caused by SARS-CoV-2, will present in many patients as a minor cold or flu, however, those with health complications – such as autoimmune diseases, asthma, heart disease and diabetes – are at greater risk of developing serious illness and adverse outcomes. And as many as 1 out of 6 patients will experience complications that could be life-threatening.
SARS-CoV-2 is thought to be a respiratory virus, primarily targeting pulmonary tissue. However, experts now agree that the virus attacks multiple critical organs and cell types including the cardiovascular system and vascular endothelial cells.
Potential therapies that address vascular system dysfunction and its sequela may have an important role in treating SARS-CoV-2 infection and its long-lasting effects. The endothelium maintains homeostasis through regulation of immune competence, inflammatory equilibrium, tight junctional barriers, hemodynamic stability, as well as optimally balanced thrombotic and fibrinolytic pathways. The novel coronavirus causes dysregulation of many of these pathways and has emerged as a mediator of severe disease.
Clinical and biomarker findings have identified how SARS-CoV-2 disrupts the immune, renin-angiotesin-aldosterone and thrombolytic balance, which are common pathways on the vascular endothelium. There are still a lot of unknowns about the novel coronavirus (SARS-CoV-2), with current knowledge mostly based on what the industry has learned about existing coronaviruses including MERS-CoV and SARS-CoV.1
After viral genome analysis, the coronavirus study group (CSG) of the International Committee on Taxonomy of Viruses concluded that the virus shares 88 percent of its genetic code with two bat-derived severe acute respiratory syndrome (SARS-like) coronaviruses. However, the study group also concluded that the sequence was more distant from SARS-CoV.2 The spikes crowning SARS-CoV-2 are typical of pneumonia in how they attach, fuse and gain entry to cells.3 Furthermore, this essentially interrupts the vascular endothelium, impacting a patient’s inflammatory response, and progressing to further complications linked to induced end-organ dysfunction such as cardiovascular disease, ARDS, vasoplegia and immune exhaustion. (See Figure 1)
As the spread and devastation of the COVID-19 pandemic continues to grow, laboratory testing plays an essential role in both diagnosis and management of patients with COVID-19. Consequently, it is vital that fast and accurate diagnostic testing strategies are implemented for effective risk stratification, monitoring of treatment efficacy and recovery.
Vascular abnormalities – dysregulation, endothelial injury and cytokine storm
The vascular endothelium plays a role in immune regulation and inflammation in which SARS-CoV-2 infection interrupts. Studies surrounding inflammation in patients with COVID-19 have strong links to cytokine storms, macrophage activating syndrome and subsequent immune exhaustion. 4
Cytokines have a vital role in the immune system and are known to be involved in the body’s response to a variety of inflammatory and infectious diseases. Overstimulation of cytokines in response to infection is known as a “cytokine storm,” a common complication of SARS-CoV-2, which strongly correlates with poor disease outcomes, including pneumonitis, acute respiratory distress syndrome (ARDS), shock, multiple organ failure, and potentially death. Many researchers have highlighted the need to identify cytokines, cytokine receptors and growth factors to classify complications where viral replication and excessive, uncontrolled systematic inflammation may lead to further complications.
The overlap in secreted cytokines in response to SARS-CoV-2 and influenza can be explained by the presence of viral RNA in the host cell’s cytoplasm during the replication cycle of both viruses, which likely induces the activation of similar intracellular anti-viral pathways and subsequent recruitment of similar immune cells to the respiratory epithelium. SARS-CoV-2’s pro-inflammatory immune signature has been likened to macrophage-activation syndrome (MAS), a life-threatening clinical entity observed in autoimmune diseases and mimicked in many viral infections, including influenza. 5
According to research estimates, cytokine storms occur in up to 5 percent of severe COVID-19 cases, with high levels of several inflammatory cytokines, including IL-6, IL-8, IL-10 and TNF-alpha. Due to the elevation of several pro-inflammatory and anti-inflammatory cytokines, a multiplex-immunoassay approach can offer several advantages over the widely utilized single ELISA tests. The simultaneous detection of multiple cytokines from a single patient sample will provide clinicians with a detailed picture and complete patient profile, facilitating a personalized approach to treatment.
Using influenza’s associated cytokine storm to derive conclusions about COVID-19’s potential virulence mechanisms poses challenges for the diagnostics industry because of SARS-CoV-2’s expanded tropism. Analysis of the medical data on patients who succumbed to COVID-19 suggest that SARS-CoV-2 infects endothelial cells to cause inflammation. Moreover, viral cytotoxicity could be playing a larger role in mediating severe COVID-19 than in influenza.
Many researchers have said this explains multi-system system organ failure and a hypercoagulable state associated with severe COVID-19, since local pulmonary endothelialitis would result in activation of the coagulation cascade and exuberant production of endothelium-derived pro-inflammatory cytokines without the need to invoke a MAS-like pathologic state. A recent study highlighted that plasma IL-6 levels in COVID-19 patients appear to be significantly lower on average (10- to 40-fold) when compared with those reported in other non-COVID-19 ARDS cohorts that display signs of a cytokine storm. These observations run counter to the hypothesis that elevated serum cytokines are driving the unprecedented morbidity and mortality observed in severe COVID-19, suggesting instead that they are consequences of local vasculopathy. 6
Associated complications – multi-system dysfunction
The SARS-CoV-2 infection has elicited a swift response by the scientific community to elucidate the pathogenesis in order to develop much needed effective therapeutics. Clinical data indicate that severe COVID-19 most commonly manifests as viral pneumonia-induced acute respiratory distress syndrome (ARDS), a clinical entity mechanistically understood best in the context of influenza A virus-induced pneumonia. Like influenza, advanced age has emerged as the leading risk factor for developing severe COVID-19. SARS-CoV-2 identified in most COVID-19 patients with underlying conditions are at a greater risk of experiencing complications that could potentially be life-threatening as they have a greater risk of developing serious illness and adverse complications.
Those illnesses and complications include ARDS, liver damage, acute kidney injury and cardiovascular issues.
Acute respiratory disease syndrome (ARDS)
Most morbidity and mortality related to COVID-19 occurs in the inflammatory phase, characterized by a dysregulated immune response and hypercoagulable state that is associated with life-threatening complications, including cardiac and renal failure, cerebrovascular disease, and ARDS.7 Strongly linked to vascular abnormalities, ARDS has widely been characterized as a noncardiogenic circulatory disorder of the lungs associated with critical illnesses such as sepsis, trauma, and immune and collagen vascular disease. The demise occurs due to progressive pulmonary hypoxia and multi-organ dysfunction syndrome (MODS) with severe inflammation.8
Much progress has been made in understanding the pathophysiology of viral pneumonia induced ARDS, particularly in the context of influenza. However, taking into context the nature of the SARS-CoV-2 virus, common links can be identified. The heterogeneity associated with COVID-19’s clinical presentation has prompted the conceptualization of novel paradigms of respiratory disease to explain the observed variability and individualize clinical management of COVID-19.9
Hepatic function
Liver damage in patients with coronavirus infections might be directly caused by the viral infection of liver cells. Patients with abnormal liver function tests are at a significantly higher risk of developing severe disease. Significantly elevated bilirubin levels, three times the upper limit, have been observed in COVID-19 patients. Other liver function markers are known to be elevated in COVID-19 patients including both AST and ALT, with markers like Albumin decreased. The presence of ACE2 receptors in the liver taken together with the local effects of systemic inflammation and possible iatrogenic toxicity seem to be the main mechanisms involved in the onset of liver damage in COVID-19 patients.10
Researchers have noted that through clinical trials and studies, SARS-CoV-2 has a negative effect on hepatic cells and is associated with other multi-organ failures. In particular, during COVID-19 progression, the liver could be involved either as a direct target of the SARS-CoV-2 (e.g. hepatocyte apoptosis or caspase-dependent pathways) and secondary to the complex pathways of systemic alterations promoted by the viral infection, mainly including inflammation and cytokine release (including IL-1, IL-6, IL-10, immune response, altered coagulation, hepatic ischemia and hypoxia, and sepsis-related abnormalities.
Renal function
The United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends that all COVID-19 patients are assessed for acute kidney injury (AKI) on admission to a hospital and their condition monitored throughout their stay. AKI is a common complication of COVID-19, especially in diabetic patients.
Serum creatinine (SCr) is the commonly utilized screening test for renal impairment; however, it is important to consider the accuracy and reliability of the method. The Jaffe and enzymatic methods are the readily available methods of SCr determination. While the Jaffe method is less expensive, it is more susceptible to interferences. These interferences can lead to the misdiagnosis of patients. Moreover, the sensitivity of SCr in the early detection of renal disease is poor, with SCr insensitive to small changes in GFR. Up to 50 percent of renal function can be lost before significant SCr levels become detectable. Cystatin C (CysC) is a superior marker of renal function and has been identified to be useful in the determination of the extent of renal damage as well as distinguishing those with severe and mild COVID-19.
Although Cystatin C is a superior marker of renal impairment, employing a multi-marker approach could identify kidney disease or injury at a much earlier stage. Using current technologies, kidney disease is typically diagnosed at around stage 4 or 5 when moderate to severe damage has already occurred. Using a multiplex approach, damage can be identified much earlier and in many cases before symptoms arise.
Cardiovascular function – the importance of Lp(a) testing
SARS-CoV-2 infection also can lead to cardiovascular manifestations in COVID-19 patients, mainly due to the interaction between the viral spike (S) protein and angiotensin-converting enzyme 2, which triggers entry of the virus into host cells. The presence of underlying cardiovascular comorbidities in patients with COVID-19 is associated with high mortality. COVID-19 can cause cardiovascular disorders, including myocardial injury, arrhythmias, acute coronary syndrome and venous thromboembolism. Many patients who present with COVID-19 have increased fibrinogen, fibrin degradation products, D-dimer and von Willebrand factor, and these elevations appear to correlate with severity of disease and thrombotic risk. Earlier reports and findings show a substantial burden of myocardial injury in patients who were critically ill or died from COVID-19.
While infection and hemodynamic stresses of acute critical illness can trigger plaque rupture and result in myocardial infarction, recent reports indicate that some COVID-19 patients show biomarker and electrocardiographic findings of myocardial infarction without evidence of acute plaque rupture on angiography. Furthermore, a recent case series reported a 78 percent prevalence of cardiovascular involvement and myocardial inflammation without apparent left ventricular impairment on cardiac magnetic resonance imaging studies of recovered COVID-19 patients without cardiac symptoms post-discharge from the hospital. This pattern of cardiac injury could result from endothelial dysfunction and coronary microvascular thrombosis in these patients, rather than coronary macrovascular thrombosis. 11
Lipoprotein(a) / Lp(a) is a strong independent marker of coronary heart-disease risk in patients with heterozygous familial hypercholesterolemia (HeFH). Lp(a) has recently been identified as a key risk marker of cardiovascular complications in COVID-19 patients. Those with either baseline elevated Lp(a) or those whose Lp(a) levels increased following infection from COVID-19, or both, may be at a significantly increased risk of developing thromboses. Consideration should be given to measurement of Lp(a) and prophylactic anticoagulation of infected patients to reduce the risk. Elevated Lp(a) levels may also cause acute destabilization of pre-existing but quiescent, atherosclerotic plaques, which could induce an acute myocardial infarction or stroke.
References
- How 2019-nCoV spreads. Centers for Disease Control and Prevention (CDC) 2020. https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html. Accessed: February 7, 2020.
- Lu R, Zhao X, Li J et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Jan 30:S0140-6736(20)30251-8. doi: 10.1016/S0140-6736(20)30251-8.
- Wang Q, Wang YH, Ma JC et al. Description of the first strain of 2019-nCoV, C-Tan-nCoV Wuhan Strain — National Pathogen Resource Center, China, 2020. http://weekly.chinacdc.cn/en/article/id/e3a460f1-661b-4180-b562-ecd8e9502082. Accessed January 6, 2021.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. Jun 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009.
- Pons S, Arnaud M, Loiselle M, Arrii E, Azoulay E, Zafrani L Immune consequences of endothelial cells’ activation and dysfunction during sepsis. Crit Care Clin. Apr 2020;36(2):401–413. doi: 10.1016/j.ccc.2019.12.001.
- Davidson S, McCabe TM, Crotta S, et al. IFNlambda is a potent anti-influenza therapeutic without the inflammatory side effects of IFNalpha treatment. EMBO Mol Med 2016; 8: 1099–1112. doi:10.15252/emmm.201606413.
- De Felice FG, Tovar-Moll F, Moll J, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the Central Nervous System. Trends Neurosci 2020; 43: 355–357. doi:10.1016/j.tins.2020.04.004.
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2: 611–620. doi:10.1016/S2213-2600(14)70097-9.
- Jain J, Gaur S, Chaudhary Y, et al. The molecular biology of intracellular events during Coronavirus infection cycle. Virusdisease 2020; 31: 1–5. doi:10.1007/s13337-020-00591-1.
- Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044.
- De Felice FG, Tovar-Moll F, Moll J, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the Central Nervous System. Trends Neurosci 2020; 43: 355–357. doi:10.1016/j.tins.2020.04.
About the Author

Martin Conway, BSc
Martin Conway, BSc, serves as Marketing Team Lead for Chemistry, Immunoassay, Molecular and Point of Care Analyzer at Randox Laboratories.