The evolution of MS as a clinical research tool
From its inception at the turn of the 20th century, MS has long been recognized as an important tool for the characterization of critical analytes, enabling the determination of the mass-to-charge ratio (m/z) resulting in elucidation of their structure. But, while the origins of MS lie in the pioneering work of physicist Sir Joseph John Thompson and chemist Francis William Aston more than a century ago, its adoption into routine clinical research labs is considerably more recent.1-4
Despite some research into the use of MS for respiratory gas analysis in the late 1950s,5 many of the earliest applications date from the 1970s, with the coupling of MS and gas chromatography (GC) for the determination of various biological analytes in urine and other biological fluids.6,7 However, as many biologically active molecules are thermolabile, polar and insufficiently volatile, GC-MS approaches typically required complex extraction and derivatization steps. As such, a lot of routine MS applications were predominantly limited to the field of forensic toxicology.
Driven by the need for improved accuracy, speed and cost-efficiency in routine clinical research laboratories, the last few decades have seen a considerable emphasis on the development of robust, reliable, accurate and sensitive LC-MS-based assays. LC is much more amenable to the separation of thermolabile and biologically active molecules than GC, and it gives analytical scientists the flexibility to analyze a diverse range of biological matrices, including blood, plasma, serum, oral fluid, urine, etc. Recent years have witnessed significant improvements in the separation resolution offered by modern high-performance liquid chromatography (HPLC) and ultra-HPLC (UHPLC) techniques, while the latest advances in column technologies, such as new biphenyl reversed phase chemistries, provide enhanced selectivity for more challenging analytes.
The demands for MS to provide accurate data for identification, confirmation of molecular identity and structure has been explored quite extensively leveraging high-resolution accurate mass (HRAM) systems. However, for routine quantitation of analytes in biological, recent advances in MS are enabling clinical laboratories to develop novel, reproducible and sensitive assays.
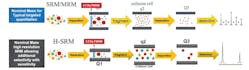
Two of the most widely used MS technologies that are efficiently leveraged for quantitation are triple quadrupole (QqQ) and HRAM systems. QqQ instruments, known for their sensitivity and selectivity, as well as their operational robustness and throughput, comprise three sets of quadrupole rods (referred to as Q1, q2 and Q3) that filter analytes based on the way their ions fragment. In typical selected reaction monitoring (SRM) experiments (Figure 1), molecular ions entering the instrument are filtered by the first quadrupole (Q1), and only those with a specific m/z may be analyzed further. A second quadrupole (q2), which acts as a collision cell, subsequently fragments the selected precursor ions to generate daughter ions, which are filtered by the third quadrupole (Q3), enabling the detection of specific fragments. Steady improvements in instrument design mean modern QqQ instruments now offer excellent reproducibility for quantitative analysis and can routinely support the sensitive determination of analytes even at picomolar concentrations in complex biological matrices.
The development of HRAM systems based on Orbitrap technology has led to considerable improvements in mass resolution, permitting the detection of incredibly small differences in the mass of analytes of interest, thereby enabling routine quantitation assays.8 Orbitrap mass analyzers conducting parallel reaction monitoring (PRM) (Figure 1) consists of three electrodes: two outer electrodes that form a barrel shape, surrounding a coaxial inner electrode, which trap ions in an orbital motion around a central axis. The m/z of the analytes can be determined by measuring the image current generated by the circular motion of individual ions. The exceptional mass resolution provided by these systems enables outstanding spectral clarity, facilitating confident mass analysis.
By combining the sensitivity, selectivity and robustness offered by quadrupoles with the outstanding mass resolution offered by Orbitrap technology ensures HRAM systems deliver impressive quantitative performance. A major difference between QqQs and HRAMs is the fact that HRAMs, in addition to PRM, can also perform full-scan analysis, enabling unknown screening and untargeted analysis of analytes and mixtures. This feature also enables retrospective data analysis, allowing analysts to ask different questions of the same sample without having to re-run tests at a later date.
Realizing the benefits of LC-MS for clinical research
The exceptional performance offered by the latest instruments means modern LC-MS techniques overcome many of the challenges associated with traditional ligand binding assays, which are still widely used by many clinical research laboratories. While these established immunoassay methods continue to be used by clinical research laboratories owing to their familiarity and ease-of-use, they require analytes to bind to specific antigens, which can be impacted by interference from molecules with similar structural characteristics. Consequently, some immunoassays can potentially lead to inaccurate or imprecise results leading to higher false negatives or false positives.
LC-MS technology, whether HRAM or QqQs, however, offers exceptional specificity and selectivity, resulting in multiple approaches to isolate specific analytes of interest from complex background matrices. QqQs in particular also offer a high degree of sensitivity, and their impressive selectivity ensures excellent signal-to-noise ratios and high analytical confidence.
Another challenge associated with immunoassays is the length of time and cost associated with implementing these tests. As immunoassays require the generation of highly selective antibodies to bind to the target analyte, it can be complex and time-consuming to develop immunoassays for some target molecules. Additionally, laboratories that are dependent on immunoassays may find they need to outsource some non-routine tests to external providers if they do not have the necessary analyte-specific reagents in-house. This can be costly and result in extended turnaround times that can ultimately delay decision-making. As LC-MS techniques do not rely on specific antigens or reagents, the methods are typically easily reproduced and replicated, regardless of the location and expertise of the user.
New LC-MS technologies designed specifically for clinical research
Despite the exceptional analytical power of LC-MS and benefits of the technique over immunoassay methods, many clinical research laboratories have yet to embrace this tool for routine analyses. LC-MS has traditionally been perceived as being an operationally complex analytical technique, and this concern is not without some foundation. LC-MS systems were initially developed with a high degree of system flexibility to enable a wide range of experiments. However, this versatility also meant that developing and optimizing routine assays using traditional LC-MS systems requires a high level of specialist technical knowledge in order to exploit its full potential. The challenge facing clinical research laboratories around recruiting and retaining trained personnel with the necessary skills and experience has therefore limited the broader adoption of LC-MS, especially when it comes to clinical research laboratories with fewer resources. Fortunately, ongoing improvements in LC-MS technologies are helping more research labs access this selective and sensitive analytical technique by automating and standardizing LC-MS-based assays.
Conclusion
From its early use for metabolite profiling to the latest clinical research assays, recent advances in LC-MS technology are enabling clinical research laboratories to address a variety of analytes with the development of robust, reliable, sensitive and reproducible assays, regardless of the type of molecules, matrix complexity and user expertise.
REFERENCES
- Chong Y-K, Ho C-C, Leung S-Y, et al. Clinical Mass Spectrometry in the Bioinformatics Era: A Hitchhiker’s Guide. Comput Struct Biotec J. 2018; 16: 316.
- Thomson JJ. On the Cathode Rays. Proc Camb Philos Soc. 1897; 9: 243.
- Thomson JJ. Rays of Positive Electricity. Proc Royal Soc Lond. 1913; A89: 1.
- Lindemann FA, Aston FW. XLVIII. The Possibility of Separating Isotopes. Lond Edinb Dubl Phil Mag. 1919; 37(221): 523.
- Fowler KT, Hugh-Jones P. Mass Spectrometry Applied to Clinical Practice and Research. Br Med J. 1957; 1(5029): 1205.
- Zlatkis A, Liebich HM. Profile of volatile metabolites in human urine. Clin Chem. 1971; 17: 592.
- Crawhall JC, Mamer O, Tjoa S, Claveau JC. Urinary phenolic acids in tyrosinemia. Identification and quantitation by gas chromatography-mass spectrometry. Clin Chim Acta Int J Clin Chem. 1971; 34: 47.
- Eliuk S, Makarov A. Evolution of Orbitrap Mass Spectrometry Instrumentation. Annu Rev Anal Chem. 2015; 8: 61.