Testing for Clostridioides difficile and its disease
For a printable version of the January CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss the role of C. difficile as an opportunistic pathogen and the challenges of diagnosing CDI versus carriers
2. Recognize the advantages and limitations of laboratory tests for CDI
3. Describe how a diagnostic approach based on an algorithm provides optimal test results
4. Discuss treatment options for CDI
C. difficile is an opportunistic anaerobic bacterial pathogen that causes diarrhea and colitis especially in hospital settings. There are other causes of hospital-acquired diarrheas (e.g., norovirus outbreaks, medications, laxatives, etc.), but C. difficile is a major cause, accounting for 5% to 15% of hospital-acquired diarrheas. Outside of C. difficile infections (CDI), the cause of many hospital-acquired diarrheas remains undiagnosed.
Variants of C. difficile continue to cause problems in our healthcare systems. The most recognized variant is ribotype 027, which appeared in the early 2000s. This ribotype caused numerous outbreaks in Europe and North America because of its resistance to flouroquinolone antibiotics. However, it now is on the decline. Treatment does not differ between infections caused by the 027 ribotype and other ribotypes. As new hypervirulent strains continue to emerge, surveillance testing is important to help identify and proactively counteract these new threats.
CDI develops in persons who have a compromised intestinal microbiota. Hospitalized elderly patients receiving antibiotics are a prime target for the disease. Antibiotics kill the microbiota, allowing C. difficile spores to germinate, grow in the intestine, and produce two very potent tissue-damaging and inflammatory toxins designated A and B that cause CDI. The spores, which persist in the patient, cause recurrent disease in about 25% of CDI cases and are difficult to eradicate from hospital environments.
A closer examination of data over the past decade suggests a shift in the epidemiology in hospital-acquired versus community-acquired CDI.1 Hospital-acquired CDI is defined as those patients who develop CDI while in a healthcare facility. Community-acquired CDI is defined as those patients who develop CDI prior to or shortly after admission to a hospital. The numbers most typically cited for CDI in the U.S. — 400,000 cases of CDI with up to 30,000 deaths — include both hospital-acquired and community-acquired cases. Lower rates of hospital-acquired CDI may be due to fewer cases caused by the hypervirulent fluoroquinolone-resistant ribotype 027, along with reduced use of fluoroquinolone antibiotics and greater attention to preventive measures. Community-acquired CDI, on the other hand, appears to be increasing, and may account for almost half of the cases of CDI, as indicated in more recently cited numbers.1
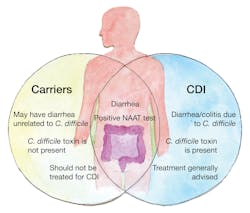
Overdiagnosis continues to be a challenge because of the large numbers of hospitalized patients who are carriers of C. difficile but who do not have CDI. Guidelines stipulate that carriers should not be treated because inappropriate treatment predisposes the patient to CDI and is not a good practice of antibioticstewardship, possibly leading to antibiotic-resistant strains. (See Figure 1)
Gold standard tests for CDI
The more than 50 laboratory tests cleared by the U.S. Food and Drug Administration (FDA) for C. difficile all have the same intended use and patient population. Importantly, results with all of these tests are to be used in conjunction with clinical history when diagnosing CDI. About half of the FDA-cleared tests are immunoassays that detect toxins A and B or glutamate dehydrogenase (GDH). Immunoassay formats include microwell-based and rapid membrane tests. NAAT (nucleic acid amplification test) assays, representing the other half, detect the toxin genes as single gene targets or as part of multiplex gastrointestinal panels.
The gold standard tests, developed about 40 years ago to help diagnose CDI, continue to be used today as comparator tests to establish performance of newer laboratory tests. The gold standard tests include:
- the cell cytotoxicity neutralization assay (CCNA) for the detection of toxin in fecal specimens
- toxigenic culture on selective media (typically cycloserine cefoxitin fructose agar [CCFA]) followed by CCNA.
CCNA detects the cell-rounding activity (i.e., cytopathic effect) of the toxins, with confirmation by neutralization with specific C. difficile antitoxin. For toxigenic culture, colonies are picked from CCFA and further tested by CCNA to confirm the isolate is toxigenic.
The gold standard tests are very accurate and sensitive. CCNA detects picograms of toxin, and CCFA can detect <100 colony-forming units per gram feces. However, both are tedious and time-consuming, requiring 2 days minimum for CCNA and 4 to 5 days for toxigenic culture. For these reasons, these tests are seldom used in today’s clinical laboratory. Instead, clinical labs have moved toward more rapid and easier-to-use formats.2Toxin Immunoassays
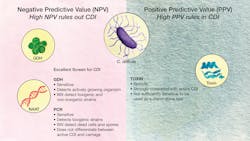
There are variant strains that produce only toxin A or only toxin B. This is why immunoassays that detect both toxins must be used since variant strains that produce only one toxin are capable of causing severe and life-threatening CDI. Toxin immunoassays exhibit higher specificity, in some cases >99%, than GDH and NAAT assays because they detect the toxins that directly damage the intestinal mucosa and trigger the inflammation that occurs in CDI. The higher specificity results in higher positive predictive values (PPV) and fewer false positive results. PPVs are affected by the prevalence of the disease in the patient population being tested. The PPVs approaching >95% with some of the better performing toxin immunoassays have been reported from healthcare facilities with prevalence rates of 5% to 15%. The lower sensitivity of toxin immunoassays, compared to the picogram amounts detected by CCNA, has raised concerns about false negative results.2 (See Figure 2)
GDH Immunoassays
GDH immunoassays detect glutamate dehydrogenase, a metabolic enzyme produced by C. difficile. GDH is an excellent biomarker for C. difficile because it is a stable enzyme produced when the organism is actively growing in the large intestine. GDH consists of six identical subunits, thus providing multiple repeating epitopes for increased binding of antibodies and better signal-to-noise ratios in immunoassays. As a result, GDH immunoassays are very sensitive with values comparable to those reported for NAAT assays for detecting the organism in fecal specimens. The high sensitivity results in high negative predictive values (NPV), and a negative GDH result accurately rules out CDI. GDH immunoassays do not differentiate between toxigenic and nontoxigenic strains. This is a limitation because nontoxigenic strains form spores and can spread in hospitals, although they are considerably less prevalent than toxigenic strains. Nontoxigenic strains do not carry the toxin genes and do not cause disease. In fact, they may be protective by outcompeting toxigenic strains in the intestine. A positive GDH result should be followed by a toxin assay when performing laboratory testing for CDI.
NAAT Assays
NAAT assays detect the toxins A and B genes (tcdA and tcdB), which are located on a large 19.6 kilobase pathogenicity locus called the PaLoc. The tests use PCR or isothermal amplification for detection. Most of the tests target tcdB although some target tcdA or both genes. A positive result confirms the presence of a toxigenic strain but does not confirm the presence of toxin. Many of the NAAT assays now available are very sensitive, detecting fewer than 50 cells per gram feces. The high sensitivity results in high NPV, and a negative NAAT result accurately rules out CDI. However, the exquisite sensitivity of the tests leads to overdiagnosis and overcalls patients who are carriers. NAAT assays will give positive results with spores and dead cells which do not cause active CDI.
Algorithm testing is recommended
C. difficile testing guidelines from the European Society of Clinical Microbiology and Infectious Disease (ESCMID), the Infectious Disease Society of America/Society for Healthcare Epidemiology of America (IDSA/SHEA), and the American Society of Microbiology (ASM) have been in place for several years.2 The most recent guideline is from the American College of Gastroenterologists (ACG).3 The ACG recommendations follow those from ESCMID and IDSA/SHEA.
The ACG guidelines note that the case numbers of CDI increased significantly when many hospitals and healthcare facilities implemented NAAT assays, and the authors raised concerns that asymptomatic patients who were colonized with C. difficile tested positive, and there are other causes of diarrhea in patients colonized with toxigenic strains. The ACG guideline note further that complications are rare in patients who are NAAT-positive but negative for toxin. For these reasons, the ACG guidelines recommend an algorithm comprised of NAAT or GDH testing coupled with toxin testing.3 These recommendations are directly in-line with those from ESCMID and IDSA/SHEA.
The IDSA/SHEA guidelines note that if specimen selection criteria are in place, the accuracy of a NAAT assay improves and recommend that a NAAT assay may be used as a stand-alone test under these conditions. Whether appropriate specimen selection procedures can be implemented in most hospital settings was a point of discussion at the recent 9th Annual International C. DIFF Conference and Health EXPO, which was held in November 2021.
Algorithm testing is considered optimal because it brings together the high NPV of GDH or NAAT assays with the high PPV of a toxin test, helping to limit false negative and false positive results, respectively. When used as the initial screen, GDH or NAAT assays accurately rule out patients who do not have CDI. This will be the majority of patients in most hospitals. Follow-up toxin testing with specimens that are GDH-positive or NAAT-positive provides the most accurate information to the physician tasked with diagnosing CDI. This approach provides confirmatory results for >90% of specimens submitted for testing. Of the remaining low number of specimens that are positive by GDH or NAAT but negative for toxin, the ESCMID guidelines define these patients as carriers, although some probably have true CDI.
Efforts have been made by several companies to produce ultrasensitive toxin tests.4 These types of tests have the potential to simplify laboratory testing and the reporting structure for CDI, assuming they can provide adequate sensitivity. However, these tests also need to exhibit high specificity to achieve the PPVs now available with higher quality FDA-cleared toxin immunoassays.
Many patients will continue to test positive for weeks or months by NAAT even after symptoms have resolved because of the persistence of spores. Resolution of symptoms more accurately correlates with the disappearance of toxin. Even so, none of the guidelines recommends a “test of cure” for CDI. Repeat testing is not routinely recommended, although on occasion, it may be performed to confirm recurrent CDI. All of the guidelines stress that the diagnosis and decision to treat is a clinical decision, and that laboratory tests “aid” but do not diagnose CDI.
CDI is an inflammatory disease
Inflammation is a hallmark of CDI. The inflammatory process is triggered by the toxins and the tissue damage they cause to the gut mucosa. Both toxins are glucosyltransferases that bind to cellular receptors and kill cells by shutting down the cytoskeletal system. In addition, both toxins trigger inflammatory mediators that result in the diapedesis of white blood cells (WBCs) into the intestinal lumen. White blood cells become activated and stimulate the release of proinflammatory mediators. The multimeric inflammasomes that develop result in additional inflammation.
The severity of the disease can be monitored by circulating WBC counts, with >15,000 per mm3 signaling severe disease. Fecal lactoferrin, a stable glycoprotein released from WBCs that migrate into the intestinal lumen, can be measured quantitatively in fecal specimens as a direct measurement of intestinal inflammation. Fecal lactoferrin and circulating WBC counts both provide laboratory results that help physicians assess CDI severity and identify patients at high risk.
The onset of sporadic irritable bowel syndrome-like symptoms (IBS) may occur after CDI.5-7 It is not uncommon for post-infectious IBS to develop after severe intestinal disease. This can happen, for example, following a norovirus infection. The triggering events in post IBS following CDI are unclear. Perhaps the persistence of spores is a complicating factor, although this has not been proven. In the absence of toxin-mediated mucosal damage, the ACG suggests that consideration of conditions “such as microscopic colitis or inflammatory bowel disease” be included as part of a clinical follow-up.3 Another possibility in the onset of post IBS is the persistence of chronic low-grade inflammation. Low grade inflammation persists in patients with inflammatory bowel disease (IBD) who are in clinical remission, and is characterized by histological damage to the colon, which is a triggering event in clinical relapse in IBD patients. Perhaps a similar situation develops during severe inflammatory CDI.
Treating CDI
The disruption of the normal diverse intestinal microbiota predisposes a patient to CDI, especially in hospital settings. Antibiotics are very good at causing this disruption, and treatment with antibiotics continues to represent the primary triggering event for CDI. Other predisposing factors are conditions such as IBD in which the intestinal microbiota is less diverse or treatment with certain medications such as proton pump inhibitors that reduce the innate protective mechanism of stomach acidity.
Mild CDI may be treated simply by stopping the inciting antibiotic and allowing the intestinal microbiota to become reestablished, an approach recommended in the 2021 ACG guideline.3 In more severe CDI, the disease typically is treated with metronidazole, vancomycin, or fidaxomicin, although vancomycin is now considered clinically superior to metronidazole. Fidaxomicin, which has a more narrow spectrum than vancomycin, is used increasingly because it has been associated with lower rates of recurrent disease.
Monoclonal antibody therapy with Bezlotoxumab (brand name Zinplava) was approved by the FDA in 2016 as a treatment for recurrent CDI in patients receiving antibiotics. The antibody, which is given as an intravenous infusion, binds to a specific epitope located in the combined repetitive oligopeptide (CROP) region of toxin B that constitutes the binding domain. Thus, protection is mediated by preventing the toxin from binding to its intestinal receptor.
Fecal transplants represent the latest efforts to treat CDI, especially in patients with recurrent CDI. The protection is based on the restoration of a more diverse intestinal microbiota capable of outcompeting C. difficile. Efforts are now underway to develop a more defined consortium of protective bacteria. The intestinal microbiota functions as a food pyramid with layers of diverse bacteria feeding off the byproducts produced by other bacteria, and it is challenging to identify specific consortia of bacteria that are protective. However, progress is being made in this area, and clinical trials are underway to establish efficacy in preventing recurrent CDI.
Patients who receive fecal transplants continued to carry C. difficile spores even though they become asymptomatic. This persistence of spores also occurs in patients following antibiotic treatment for CDI. Recommendations in Europe and the U.S. include antibiotic therapy for initial onset of CDI and consideration of fecal transplants in patients with recurrent disease.
CDI during COVID-19 pandemic
The Fall 2021 surge of the SARS-CoV-2 Delta variant overwhelmed hospitals, and resources such as laboratory instrumentation, reagents, and plastics have been limited. For these reasons, labs have looked for alternative ways to make testing more flexible and improve lab workflow. Modified approaches such as rapid testing for CDI have helped lower the burden.
During the early stages of the COVID-19 pandemic, antibiotics were heavily overused to treat COVID-19 patients due to the lack of alternative treatments and because of concerns about secondary bacterial pneumonias often seen with influenza illnesses. This overuse raised concerns about increased rates of CDI. By mid-2020, antibiotic overuse had been curtailed because secondary pneumonias were not being seen in COVID-19 patients at the levels seen, for example, following flu outbreaks. Although little data has been reported on rates of CDI in COVID-19 patients, it is no surprise that mitigation efforts against COVID helped reduce the incidence of CDI and other hospital-acquired infections.
Many COVID-19 patients, perhaps up to 25%, develop diarrhea.8 The association of respiratory viruses with intestinal disease is not a new observation and has been reported, for example, with influenza virus. The SARS CoV-2 ACE2 receptor is present in high numbers in the intestine, so the binding of the virus to its intestinal receptor likely causes diarrhea due to cellular damage. SARS CoV-2 and other respiratory viruses appear to lower the diversity of the intestinal microbiota, raising the question of how this occurs and whether virus-induced dysbiosis is a predisposing factor for CDI.9-11
Conclusions
- The burden of CDI on healthcare continues to be a major problem, and elderly hospitalized patients treated with antibiotics continue to be a primary susceptible population. Rates of hospital-acquired CDI are decreasing, but community-acquired cases continue to increase due to spores in the environment, exposure in healthcare facilities, and variants.
- There are numerous tests available in immunoassay and NAAT formats. Each offers advantages and each has limitations. An algorithm approach brings together the advantages of these tests and helps physicians establish an accurate diagnosis when used in conjunction with patient history. This approach results in optimal patient healthcare, minimizes overdiagnosis, and represents good antibiotic stewardship.
- With the epidemic strain 027 that appeared in the early 2000s, fluoroquinolone resistance developed following a single mutation in the gyrase gene. Variants of C difficile, such as the toxin B-only variant causing outbreaks in Asia, continue to be problematic. Surveillance efforts need to continue to identify variants, determine their involvement in CDI, and ensure that they are accurately detected in laboratory tests.
- Inflammation is a primary reason why CDI becomes severe. Elevated white blood cell counts and elevated fecal lactoferrin, a marker of intestinal inflammation, indicate severe CDI. Elevated levels of either marker are associated with worse clinical outcome.
- SARS-CoV-2 replicates in the intestine and is present in fecal specimens from COVID-19 patients. This respiratory virus binds to ACE2 intestinal receptors, causes diarrhea, and has been associated with co-infections with C. difficile, leading to questions about the etiology of intestinal disease in these patients.
- Testing strategies that minimize overdiagnosis of CDI will result in appropriate treatment for the patient. Treating carriers can lead to patient harm and has been associated with an increased prevalence of antibiotic resistance, including VRE.12 Minimizing overdiagnosis represents good antibiotic stewardship and helps to prevent the evolution of antibiotic-resistant strains.
References
- Guh AY,Mu Y,Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;(14) 382:1320–30. doi: 10.1056/NEJMoa1910215.
- Lyerly DM, Boone JH, Carman RJ, et al. Clostridioides difficile the challenge, tests, and guidelines. ACS Infect. Dis. 2020;6(11):2818-2829. doi: org/10.1021/acsinfecdis.0c00290.
- Kelly C, Fischer M, Allegretti J, et al. ACG Clinical Guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021; 116(6):1124-1147.: doi: 10.14309/ajg.0000000000001278.
- Kraft C, Mehta N. Is the ultrasensitive toxin immunoassay the solution to the goldilocks problem of Clostridioides difficile diagnostics? Oxford University Press, Infectious Diseases Society of America. Clin Infect Dis. 2021 Sep 19;ciab833. doi: 10.1093/cid/ciab833 .
- Guti´errez RL, Riddle MS, Porter CK. Increased risk of functional gastrointestinal sequelae after Clostridium difficile infection among active duty United States military personnel (1998–2010). Gastroenterology. 2015;149(6):1408–14. doi: org/10.1053/j.gastro.2015.07.059.
- Wadhwa A, Al Nahhas MF, Dierkhising RA, et al. High risk of postinfectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol Ther. 2016;44(6):576–82. doi: 10.1111/apt.13737.
- Tariq R, Weatherly R, Kammer P, etc. Experience and outcomes at a specialized Clostridium difficile clinical practice. Mayo Clin Proc Innov Qual Outcomes. 2017;1(1):49-56. doi: 10.1016/j.mayocpiqo.2017.05.002.
- Guo M, Tao W, Flavell R, et al. Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat Rev Gastroenterol & Hepatol. 2021; 8(4):269-283. doi: org/10.1038/s41575-021-00416-6.
- Burchill E, Lymberopoulos E, Menozzi E, et al. The unique impact of COVID-19 on human gut microbiome research. Front Med. 2021;8:652464. doi: 10.3389/fmed.2021.652464.
- Lakkasani S, Chan K, Shaaban H. Clostridioides difficile in COVID-19 patients, Detroit, Michigan, USA, March-April 2020. Emerg Infect Dis. 2020;26(9):2299-2230. doi: org/10.3201/eid2609.202505.
- Sandhu A, Tillotson G, Polistico J, et al. Clostridioides difficile in COVID-19 patients, Detroit, Michigan, USA, March-April 2020. Emerg Infect Dis. 2020;26(9):2272-2274. doi: org/10.3201/eid2609.202126.
- Fishbein S, Hink T, Reske K, et al. Randomized controlled trial of oral vancomycin treatment in Clostridioides difficile-colonized patients. mSphere. 2021;6(1):e00936-20. doi: org/10.1128mSphere00936-20.
About the Author

Jodie Y. Lee, MS, MBA
serves as Marketing Manager for TECHLAB. She has spent 16 years in the life science and diagnostic industries.

David M. Lyerly, PhD
is a Co-Founder and serves as Chief Science Officer for TECHLAB, Blacksburg, VA, which manufactures a variety of in vitro diagnostic tests for enteric diseases.