The current state of SARS-CoV-2 antibody tests and their future potential uses
For a printable version of the December CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Name the most effective testing methods for diagnosing current SARS-CoV-2 infection.
2. Compare and contrast how many days after infection IgG, AgA, and IgM antibodies can be detected for SARS-CoV-2.
3. Discuss possible causes of false positive and false negative test results.
4. Discuss when monoclonal antibody therapy is best used for SARS-CoV-2 patients.
Serological tests have long been a bellwether for the clinical laboratory evaluation of infectious diseases but to date have played only a very limited role in the evaluation of SARS-CoV-2 infections. Here, we discuss the current state of SARS-CoV-2 antibody (Ab) tests and their future potential uses.
Human antibody response to infection with SARS-CoV-2
Almost all immunocompetent individuals mount an antibody response to SARS-CoV-2 infection,1 and 90% of individuals that do mount an antibody response develop detectable, neutralizing antibodies to SARS-CoV-2.2 Asymptomatic individuals may seroconvert later in the course of infection, or not at all, and have lower IgG levels compared to symptomatic patients, suggesting that asymptomatic patients may mount a weaker response, and titers may diminish earlier.3,4
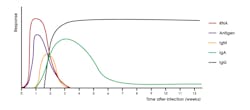
These findings, however, may not directly correlate with immune status as individuals with asymptomatic SARS-CoV-2 infection were found to mount vigorous T cell responses.5 Other studies have shown that disease severity modulates the magnitude but not the kinetics of the antibody response.6 Antibodies resulting from SARS-CoV-2 infection are generally detectable within 10-14 days post symptom onset (Figure 1).6-9 A study profiling early humoral response demonstrated that IgA and IgM antibodies can be detected as early as five days after infection, with levels increasing in the second and third weeks.10,11
IgG antibodies were shown to be detectable later, around 7-14 days after symptom onset.10,11 The results of a study by Seow J, Graham C, Merrick B, et al, suggest that most individuals ultimately seroconvert with an IgG, IgM, and IgA response, but a subset of individuals were demonstrated to seroconvert with only an IgG, IgM, or IgA response.6 IgM and IgA responses tend to decrease fairly rapidly with IgG responses being much more durable over time (Figure 1).2,6 Antibodies to SARS-CoV-2 are known to persist for months following infection, but levels do wane over time.2,6,12,13 However, the clinical significance of this finding is not fully understood, as a minimum threshold of antibody necessary to confer immunity has not been established. Vaccination is known to be efficacious at mitigating SARS-CoV-2 infection and in preventing severe disease.14-16 Furthermore, convalescent serum is known to be an effective therapy for individuals with severe COVID-19 disease.17,18 Collectively, these findings suggest that an antibody response to SARS-CoV-2 plays an important role in modulating COVID-19 disease.
Current FDA EUA offering for SARS-CoV-2 Ab assays
The U.S. Food and Drug Administration (FDA) performed independent studies to evaluate the clinical performance of SARS-CoV-2 Ab tests. These studies resulted in the withdrawal or amendment of the emergency use authorization (EUA) for several tests because the assays’ clinical performance did not meet FDA requirements. False positives can result from cross reactivity with preexisting antibodies from previous infection with other coronaviruses that cause the common cold (229E, NL62, OC43, and HKU1), SARS-CoV, or MERS-CoV, as well as with antibody tests with low specificity.19 Negative results may also occur in SARS-CoV-2 infected individuals due to lack of antibody production in the early stages of infection, and thus, making serology tests not suitable for diagnosis of infection.19,20
All SARS-CoV-2 Ab tests that are currently authorized by the FDA under EUA are intended to aid in the detection of recent or past infection indicating an adaptive immune response.21 A comprehensive list of serology tests that have been granted EUA can be found on the FDA website.
Two different types of serology tests have been granted EUA, binding antibody tests and neutralizing antibody (nAb) tests.22 Binding antibody tests detect the presence of antibodies to the SARS-CoV-2 virus, but the antibodies detected are not necessarily capable of conferring immunity.22 Binding antibody tests employ several different types of assay methodologies, including lateral flow (LF), microfluidic immunoassay, chemiluminescence (CIA), and enzyme linked immunosorbent assay (ELISA). LF and microfluidic methods are rapid, compact, portable, easy to use, and typically require only a small amount of blood (fingerstick), serum, or plasma. Results are generally reported qualitatively. These tests lend themselves well for use in the field or at the point of care by non-laboratory medical staff. However, the performance characteristics (especially sensitivity) differ among many LF and microfluidic immunoassays.
CIA and ELISA methods are laboratory-based methods best suited for the high throughput setting of the central laboratory. Results can be reported qualitatively, semi-quantitatively, or quantitively, depending on their EUA intended use. These methods require either plasma or serum samples and are performed in a moderate- or high-complexity laboratory setting.
Neutralizing antibody (nAb) tests detect antibodies that bind to a specific part of the SARS-CoV-2 virus and have been observed to prevent infection by SARS-CoV-2 in vitro.21 Neutralizing antibody tests are required to be performed in a high complexity clinical laboratory. There are three types of nAb tests: Virus neutralization test (VNT), Pseudovirus neutralization tests (pVNT), and Competitive neutralization tests (cVNT).21,22 Currently, only one neutralizing antibody test (cVNT) has received an FDA EUA for the qualitative detection of nAbs.21
Additionally, the FDA requires SARS-CoV-2 Ab test developers to continually monitor for new and emerging viral mutations and variants, as well as their potential impact on test performance.23 For any viral mutations or variants that are identified as prevalent and/or clinically significant, test developers should assess whether the amino acid change(s) in the viral proteins are critical to their test design. If so, these mutations and variants should be evaluated using clinical specimens to assess the impact of the mutation or variant on test performance. The FDA states that the aggregate impact of the mutations should not reduce the clinical performance of a test by 5% or more or decrease the clinical performance point estimates for the test below the clinical performance recommendations.23
SAR-CoV-2 Ab assays target antigens
Different SARS-CoV-2 Ab assays detect antibodies to different major viral antigens, such as the SARS-CoV-2 nucleocapsid (N) protein, spike (S) protein (including subunits S1 and S2), and RBD (receptor binding domain) of the S protein.24 The N protein, an immunodominant protein, is highly conserved among the CoV genus and is found within the virus on the nucleocapsid.21,25 Facilitating viral entry into host cells, the S protein is located on the surface of virus, protruding from the envelope, and is comprised of three segments: the ectodomain, single-pass transmembrane, and intracellular tail.26 The ectodomain consists of the S1 subunit, containing the RBD, and the S2 subunit.26
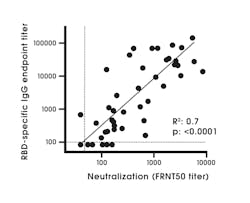
An antigen target(s) is an important factor when evaluating a test, as different antigen targets are known to be associated with differing assay performance characteristics. Studies have shown that N and S1 ELISAs detect SARS-CoV-2 antibodies with high specificity, in comparison to the full length S protein due to the S2 subunit being highly conserved among coronaviruses compared to S1.19,27 Increased cross reactivity has been observed with N and other non-S targets compared to S1 targets.9 The RBD protein has shown high sensitivity and specificity for antibody detection.28 The RBD antibody has a strong association with SARS-CoV-2 neutralizing antibodies (Figure 2).28 The region of the RBD is poorly conserved among coronaviruses and is known to be a major target of human antibodies.29
A study published in Science Immunology in June 2020 concluded that utilizing recombinant SARS-CoV-2 RBD antigen for the detection of antibodies induced by SARS-CoV-2 is highly sensitive and specific.29 Antigen targets also influence the clinical information that can be rendered by a specific SARS-CoV-2 Ab test. For example, the majority of individuals that recover from infection with SARS-CoV-2 elicit antibody responses to N, S, RBD and other proteins. 6,21 In contrast, because all current EUA COVID-19 vaccines utilize only mRNA sequences encoding for the SARS-CoV-2 S protein,14-16 vaccinated individuals (that have not had a previous infection with SARS-CoV-2) have been observed to only have antibodies to the SARS-CoV-2 S and RBD proteins.21
SARS-CoV-2 Ab assays and isotype detection
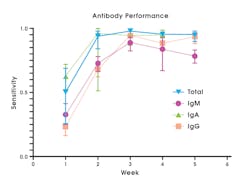
SARS-CoV-2 Ab tests collectively can detect a variety of immunoglobulin isotypes or isotype combinations. There are tests that detect individual antibody isotypes (IgG or IgM, or IgA) and tests that detect a combination of total antibody either IgG/IgM or IgG/IgM/IgA, where no differentiation of the specific isotype is made.20 IgM tests typically have lower sensitivity to detect past infection compared to IgG or total antibody (Figure 3).20
Tests designed to detect and differentiate between IgM and IgG in combination and IgA typically have lower specificity than IgG or total antibody.20 Combined IgG/IgM assays are more sensitive than assays that measure either antibody separately.9 To have substantial clinical utility, a serology test must be both highly sensitive and specific.20
Total antibody or IgG tests are preferred due to increased sensitivity (Figure 3) and specificity, compared to IgG/IgM, IgM, or IgA tests.20 Compared to other antibody classes, detection of IgG or total antibody at 3-4 weeks post symptom onset provides highest sensitivity and specificity, leading to lower false negative and false positive result rates.20 Total antibody tests that include IgA may have increased clinical utility, since at least one study has shown that a subset of SARS-CoV-2 infected individuals seroconverted with only an IgA response.6
The FDA has set minimal performance standards for SARS-CoV-2 Ab assays using PCR as the comparator. For tests that detect and differentiate IgM and IgG antibodies, clinical agreement studies should demonstrate an overall combined positive percent agreement (PPA) and negative percent agreement (NPA) of at least 90% and 95% respectively, and IgM PPA of at least 70%.23 Tests that detect total antibody, only IgG or only IgM, should demonstrate a minimum PPA of 90% and NPA of 95%.23
Detecting previous exposure
Qualitative antibody assays represent a cost effective and easily implemented way to determine if an individual has been exposed to SARS-CoV-2. The presence of antibodies is indicative of an adaptive immune response resulting from previous exposure. Thus, a positive SARS-CoV-2 antibody test would be expected for individuals who have had a recent or past infection. To date, all authorized SARS-CoV-2 Ab assays have received EUA with this indication for use.
In addition, there are numerous other situations in which Ab assays could be useful in providing information for clinical decision-making, but these use cases have not received authorization or approval by the FDA.
Triaging individuals with severe disease for tMab treatment
Monoclonal antibody (tMab) infusions have received FDA EUAs to treat COVID-19 in non-hospitalized COVID-19 patients30 and are administered as post-exposure prophylaxis for individuals who are at high risk for severe disease and who have not been vaccinated, or are expected to have an inadequate immune response to SARS-CoV-2 vaccination.31-33 Monoclonal antibody therapy administration is time sensitive and must start as soon as possible post exposure or within 7 days.31 The presence of SARS-CoV-2 antibodies in these patients could be indicative of an adequate immune response from previous exposure or vaccination. Therefore, individuals with detectable antibodies would not be candidates for tMab treatment. There are ongoing studies to examine whether these individuals should be excluded as candidates. Highly sensitive and specific SARS-CoV-2 Ab assays could identify the presence of pre-existing antibodies in candidate patients for tMab therapy. Performing these tests at the point of care could be used to rapidly inform a clinician’s decision whether to administer therapy. Given that tMab therapy must be administered as soon as possible post-exposure or within a maximum of 7 days,31 a point of care SARS-CoV-2 Ab test solution has the potential to prove optimal.
Assessing seroconversion post vaccination
Vaccines that have received EUA or are in development aim to elicit neutralizing antibodies against SARS-CoV-2.21 The majority (but not all) antibodies to RBD are neutralizing.34 Neutralizing antibodies to antigens other than RBD tend to recognize the S protein.34 Therefore, antibody tests that utilize RBD and S antigen targets could prove useful for detecting seroconversion post vaccination.21 Currently, no SARS-CoV-2 Ab test has been authorized by the FDA for this intended use and the FDA does not recommend use of antibody testing for assessing immunity post-vaccination. Studies are currently underway to evaluate SARS-CoV-2 antibody tests for the assessment of post vaccination seroconversion.
Differentiate SARS-CoV-2 previous infection and vaccination
Human infection with SARS-CoV-2 results in antibody development against many different viral proteins, including N, S, and RBD.21 Since vaccination-induced immunity results in development of antibodies to only the S and RBD, serology tests could potentially be used to differentiate natural infection from vaccination. A vaccinated person testing positive for antibody against vaccine targets (S or RBD), and negative for other antigens (N), suggests that they have vaccine-induced antibody production and were never infected with SARS-CoV-2.21 Conversely, a vaccinated person testing positive for antibodies other than antibodies against vaccine targets, such as the N protein, suggests recent or past SARS-CoV-2 infection that could have occurred before or after vaccination.21 An unvaccinated individual testing positive by any SARS-CoV-2 Ab test could, therefore, be indicative of recent or past infection.21 To date, no SARS-CoV-2 Ab test has been authorized by FDA for this purpose, and the clinical utility of differentiating specific antibody response elicitors remains unelucidated.
Determining immunity status and need for booster
SARS-CoV-2 Ab tests will also likely play a role in the development of future vaccines by assessing efficacy of vaccines in clinical trials.35 For effective monitoring of post-vaccination efficacy and prediction of immune status, SARS-CoV-2 Ab tests need to provide a quantitative result, detect the types of antibodies capable of conferring immunity (ex. neutralizing antibodies), and the test result must be correlated to a threshold cut-off that is known to confer immunity.39,40 Clinical outcome studies to determine if universal antibody threshold can be established to indicate whether an individual is immune to infection or reinfection have not yet been completed. Such studies would need to include both SARS-CoV-2 infected and noninfected individuals who have or have not been vaccinated. As such, no existing SARS-CoV-2 Ab test can predict immunity status.
Researchers at Sinai Hospital in Baltimore are conducting two soon-to-be-published studies of point-of-care serology tests to aid in determining a patient’s immune status.37 The researchers say this testing may be the easiest, fastest way to determine if someone needs a booster shot.37 However, research regarding this is still ongoing. Paul Gurbel, MD, Director of the Sinai Center for Thrombosis Research and Drug Development, stated that the tests can help doctors determine which patients might need a booster and when they would need it.38
The presence of neutralizing antibodies post exposure or vaccination indicates protection from infection; however, cut-off levels for neutralizing antibodies have yet to be determined.9 It is conceivable that using serology tests at the point of care could identify individuals with inadequate immune responses to vaccination or infection to help inform whether to administer a vaccine or booster. However, more studies are required to establish clinical utility.
Summary
Currently, only tests authorized for use by the FDA should be used for clinical diagnostic purposes. SARS-CoV-2 Ab tests with the highest sensitivity and specificity are preferred.21 To maximize the clinical utility and full potential of SARS-CoV-2 antibody testing, it is likely that methods will need to be capable of rendering a quantitative test result, detect antibodies that are capable of conferring immunity (ex. neutralizing antibodies), and, most importantly, results must correlate with clinical outcome studies. In context, SARS -CoV-2 Ab tests have the potential to one day aid in identifying the presence or absence antibodies to indicate when therapies and vaccines are administered, and to effectively assess an individual’s immunity status.
References:
- Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033-1036. doi:10.1038/s41591-020-0913-5.
- Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227-1230. doi:10.1126/science.abd7728.
- Zhang Yongchen, Han Shen, Xinning Wang, Xudong Shi, Yang Li, Jiawei Yan, Yuxin Chen & Bing Gu (2020) Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients, Emerging Microbes & Infections, 9:1, 833-836, DOI: 10.1080/22221751.2020.1756699.
- Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. doi:10.1038/s41591-020-0965-6.
- Patricia Kaaijk, Verónica Olivo Pimentel, Maarten E. Emmelot, Martien Poelen, et al. Children and adults with mild COVID-19 symptoms develop memory T cell immunity to SARS-CoV-2 medRxiv 2021.09.10.21263333. doi: https://doi.org/10.1101/2021.09.10.21263333.
- Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5(12):1598-1607. doi:10.1038/s41564-020-00813-8.
- Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020;101(8):791-797. doi: 10.1099/jgv.0.001439..
- Ramachandran T, Chattopadhyay S, et al. Longitudinal serology of SARS-CoV-2-infected individuals in India: A prospective cohort study. Am J Trop Med Hyg. 2021 May 18;105(1):66-72. doi: 10.4269/ajtmh.21-0164.
- Ong D, Fragkou P, Schweitzer V, et al. How to interpret and use COVID-19 serology and immunology tests. Clin Microbiol Infect. 2021;27(7):981-986. doi:10.1016/j.cmi.2021.05.001.
- Guo L, Ren L, Yang S, Xiao M, et al, Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020; 71(15): 778–785. doi: 10.1093/cid/ciaa310.
- Ma H, Zeng W, He H. et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020. 17(7):773–775. doi: 10.1038/s41423-020-0474-z.
- Beaudoin-Bussières G, Laumaea A, Anand SP, et al. Decline of humoral responses against SARS-CoV-2 Spike in convalescent individuals. mBio. 2020;11(5):e02590-20. doi:10.1128/mBio.02590-20.
- Crawford et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;223(2):197-205. doi: 10.1093/infdis/jiaa618.
- Johnson and Johson. Janssen COVID-19 Vaccine Package Insert. https://www.janssencovid19vaccine.com/hcp/covid19-resources.html. Revised August 27, 2021. Accessed October 29, 2021.
- Moderna. Moderna COVID-19 Vaccine Package Insert. https://www.modernatx.com/covid19vaccine-eua/providers/. Revised August 27, 2021. Accessed October 29, 2021.
- Pfizer. Pfizer-Biontech COVID-19 Vaccine Package Insert. https://www.pfizer.com/products/product-detail/pfizer-biontech-covid-19-vaccine. Revised September 22, 2021. Accessed October 29, 2021.
- Wang X, Guo X, Xin Q, et al. Neutralizing antibody responses to severe acute respiratory syndrome Coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020;71(10):2688-2694. doi:10.1093/cid/ciaa721.
- Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619-629. doi:10.1056/NEJMoa2031304.
- Chau, C.H., Strope, J.D. and Figg, W.D. (2020), COVID-19 Clinical diagnostics and testing technology. Pharmacotherapy. 2020 Aug;40(8):857-868. doi: 10.1002/phar.2439.
- Hanson KE, Caliendo AM, Arias CA, Englund JA, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Serologic Testing. Infectious Diseases Society of America 2020; Version 1.0.0. https://www.idsociety.org/practice-guideline/covid-19-guideline-serology/. Accessed August 10, 2021.
- Centers for Disease Control and Prevention. Interim guidelines for COVID-19 antibody testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Updated September 21, 2021. Accessed October 13, 2021.
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA authorizes first test that detects neutralizing antibodies from recent or prior SARS-CoV-2 infection. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-test-detects-neutralizing-antibodies-recent-or. Updated November 6, 2020. Accessed October 13, 2021.
- U.S. Food and Drug Administration. In vitro Diagnostics EUAs. FDA- Serology Template for Test Developers. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas. Accessed: October 13, 2021.
- U.S. Food and Drug Administration. In Vitro Diagnostics EUAs — Serology and other adaptive immune response tests for SARS-CoV-2. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-serology-and-other-adaptive-immune-response-tests-sars-cov-2. Accessed October 13, 2021.
- Bai Z, Cao Y, Liu W, Li J. The SARS-CoV-2 nucleocapsid protein and its role in viral structure, biological functions, and a potential target for drug or vaccine mitigation. Viruses. 2021; 13(6):1115. https://doi.org/10.3390/v13061115.
- Mittal A, Manjunath K, Ranjan RK, Kaushik S, Kumar S, Verma V (2020) COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog 16(8): e1008762.
- Okba N, Müller MA, Li W, et al. Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients. Emerging Infectious Diseases. 2020;26(7):1478-1488. doi:10.3201/eid2607.200841. doi: 10.1371/journal.ppat.1008762.
- Suthar M, Zimmerman M, Kauffman R, Ahmed R, Rodback J, et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020 Jun 23;1(3):100040. doi: 10.1016/j.xcrm.2020.100040.
- Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 2020;5(48):eabc8413. doi: 10.1126/sciimmunol.abc8413.
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA authorizes additional monoclonal antibody for treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19. Updated May 26, 2021. Accessed: October 13, 2021.
- McInstosh K. COVID-19: epidemiology, virology, and prevention. UpToDate.https://www.uptodate.com/contents/covid-19-epidemiology-virology-and-prevention. Revised October 6, 2021.Accessed October 29, 2021.
- U.S. Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of REGEN-COV. Last Revised: 09/17/2021. https://www.fda.gov/media/145611/download. Revised September 17, 2021. October 29, 2021.
- National Institutes of Health. : Covid Treatment Guidelines: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/. Updated October 27, 2021. Accessed October 13, 2021.
- Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369(6504):650-655. doi:10.1126/science.abc6952.
- Heffernan E, Hannan MM, Fitzgibbon M (2021) The role of SARS-Cov-2 antibody testing; current and future applications. J Infect Dis Ther. 9(4):466. doi: 10.4172/2332-0877.1000466
- Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423-4428. doi:10.1016/j.vaccine.2021.05.063.
- LifeBridge Health. Research on rapid test to detect COVID-19 antibody levels. News Wise. August 18, 2021. https://www.newswise.com/coronavirus/research-on-rapid-test-to-detect-covid-19-antibody-levels/?article_id=756067. Accessed October 29. 2021.
- Robinson, L. Rapid test study could help doctors determine who needs COVID-19 booster shot. WBALTV11. Updated: August 19, 2021. https://www.wbaltv.com/article/rapid-test-study-covid-19-booster-shot/37351507#. Accessed October 29, 2021.
About the Author

Jared Gresh, MT(ASCP)
is a Technical Implementation Specialist at LumiraDx. He is a graduate of the medical technology program at Salve Regina University,

Brian K. DuChateau, PhD, D(ABMLI)
is a board-certified clinical laboratory immunologist who received his PhD in immunology and medical microbiology from the University of University of Wisconsin-Madison and completed a post-doctoral residency in clinical immunology at the Chicago Medical School. With more than 20 years of experience as a clinical laboratory director and 12 years of in vitro diagnostics (IVD) experience, he currently serves as the LumiraDx Vice President of U.S. Scientific and Clinical Affairs.