Advances in colorectal cancer therapeutic biomarkers
For a printable version of the October CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the current standards of care regarding tests and treatments for colorectal cancer.
2. Differentiate how immune therapies can impact colorectal cancer.
3. Discuss a novel approach using extracellular vesicles (EV’s) to sensitize CRC to immunotherapy treatments.
4. List the biomarkers associated with colorectal cancer.
Colorectal cancer (CRC) remains a significant public health burden in the U.S., estimated to be the fourth leading cause of cancer diagnosis but the second leading cause of deaths in 2021.1 This mortality deficit is driven largely by the limited treatment options and poor disease control seen in the advanced stages of cancer, where chemotherapy is still the primary treatment modality.
Insights into molecular subtyping of cancers including colon, breast, and prostate, along with the discovery of so-called ‘driver’ mutations, have significantly advanced targeted and immune therapies for these cancers. Yet, the success of targeted therapies in CRC remains limited, and their utility remains primarily as an adjunct to mainline chemotherapy, or as a salvage treatment, which is a therapy used after others have failed to produce results.
However, a number of recent clinical trials have sought to change that, bringing targeted and immune therapies into earlier lines of treatment. In addition, a number of recent translational studies have demonstrated promising early results in the sensitization of CRC to existing targeted or immune treatments. Through sensitization, tumors that historically would not respond to immunotherapy treatments may be converted to responders. Given the durability of response seen in most immune therapies, this represents a significant therapeutic promise.
In this article, we will review the current standards of care regarding tests and treatments for therapeutic biomarkers, with particular attention to immune therapies, including a novel approach, which uses extracellular vesicles (EV’s), or lipid bound vesicles secreted into extracellular space by cells, to sensitize CRC to immunotherapy treatments.
Standard of care testing for therapeutic biomarkers
As with all cancers, the selection of a standard treatment for CRCs depends largely on the clinical and pathological stage. Treatment for early-stage colon and rectal cancers, which are potentially curable, relies on local therapies, including surgery and radiation. In later-stage cancers, providers also may add chemotherapy or targeted therapies.
Biomarker testing is of limited utility in localized colon cancer, with only microsatellite instability (MSI) testing recommended. MSI occurs when there is a cellular change to the number of repeated DNA bases in a microsatellite, or a repeated DNA sequence, from how it was originally inherited. Knowing whether a cancer has MSI helps when forming a treatment plan. For example, MSI testing is used primarily as a screening tool for Lynch Syndrome, which is the leading cause of hereditary colorectal cancer. 2
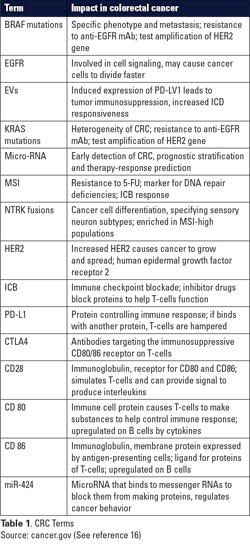
Much broader testing is recommended in the unresectable or metastatic setting, where MSI status, along with mutations in the genes KRAS and BRAF are evaluated, as are amplifications of the gene HER2, or fusions of the NTRK gene. Of these biomarkers, KRAS, NTRK, and MSI have all been associated with immunomodulation or immunotherapy response, making them promising targets for therapy for patients with metastatic colon cancer. (See Table 1.)
KRAS mutations
KRAS testing has long been a prerequisite to the treatment of advanced CRC, primarily due to its relationship with the EGFR (Epidermal Growth Factor Receptor), a protein, tyrosine kinase, that binds to the epidermal growth factor and is involved in cell signaling pathways for cell division. Mutations in the EGFR gene cause proteins to be made more than normal on some cancer cells, which causes the cancer cells to divide faster. The KRAS gene creates a protein involved in cell signaling; thus, mutations to the KRAS gene may also cause cancer cell growth. KRAS mutations are among the most common alterations found in CRC.3
Anti-EGFR antibodies, like cetuximab, have been shown to be effective in combination with chemotherapy in the treatment of advanced CRC.4 KRAS mutations in CRC tend to be constitutively activating; meaning, upstream EGFR inhibition has no effect on its signaling, rendering anti-EGFR antibodies ineffective in the treatment of KRAS mutant tumors.
Thus, for over a decade, KRAS has played a key role as a predictive biomarker in colorectal cancer treatment.
More recent studies have looked to expand the role of immunotherapies by allowing direct targeting of mutant KRAS. Multiple pre-clinical studies, along with early phase clinical trials, have demonstrated the efficacy of inhibitors of specific KRAS mutants in multiple solid tumor types.5 With the recent approvals by the U.S. Food and Drug Administration (FDA) of KRAS G12C mutant inhibitors, such as sotorasib, in lung cancer, and preliminary evidence of efficacy against this same mutation in CRC, targeted therapies for KRAS-mutant CRC may soon become the standard of care.
NTRK fusions
NTRK fusions take place when a piece of the chromosome with the NTRK gene breaks off and joins with a different gene on another chromosome, leading to abnormal proteins known as TRK fusion proteins, which cause cancer growth. This is also known as neurotrophic tyrosine receptor kinase gene fusion.
Fusions of NTRK are much rarer in CRC, compared with other types of cancer, comprising less than 1% of advanced cancer cases. Recently, the FDA approved a highly efficacious inhibitor for use in all cancer types where this fusion is found.6 Though NTRK mutations are considered to be mutually exclusive with KRAS, there are some data to suggest that RAS protein mutations may be a potential resistance mechanism to NTRK fusion inhibition.
Biomarkers and immunotherapy
Of note, NTRK fusion-positive CRC appears to be enriched for microsatellite instability (MSI-high) and a high tumor mutational burden (TMB-high).7 NTRK fusions are also enriched within the MSI-high population, though the rarity of both biomarkers leaves the number of studied patients low.8 Further, several studies have demonstrated that RAS protein mutation leads to immunosuppression and reduced immune infiltration.9 Thus, a greater understanding of the tumor immune environment and immunotherapy, in general, may aide in treatment of multiple biomarker-driven diseases.
MSI and immunotherapy
Testing for microsatellite instability, a marker for DNA repair deficiencies and a CRC-specific immune checkpoint blockade (ICB) response, is currently recommended for all stages of colorectal cancer. Immune checkpoint inhibitors are drugs that block the proteins involved in regulating the checkpoints of the immune cascade, which are triggered by some cancer cells to suppress immune responses and inhibit T-cells from killing cancer cells. Blocking these checkpoints helps T-cells function.
While TMB-high status is also an approved indication for ICB treatment in solid tumors, the response rates for TMB-high patients in CRC is poor, ranging from 4-14%, and testing is not recommended by the National Comprehensive Cancer Network (NCCN). Yet, even within the MSI-high patient population, response rates to ICI therapy can be below 50%. Further complicating the picture, one recent study suggests that a subset of heavily pretreated metastatic microsatellite stable (MSS) colorectal cancer, without liver metastases, may also respond to ICB therapy.10
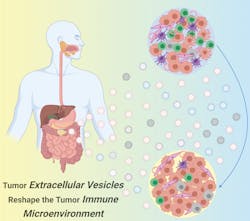
Given these conflicting data, and mediocre ICB response rate in MSI-high CRC, significant efforts are being made to understand what aspects of the tumor microenvironment may affect ICB treatment response. Multiple recent studies have suggested that extracellular vesicles (EVs), lipid-bilayer particles involved in numerous physiologic processes, could be utilized to induce ICB response.11, 12, 13, 14 In particular, the work by Zhao and colleagues, evaluating the role of EVs in modulated T-cell and ICB response, represents a promising new avenue of investigation.
Extracellular vesicles and the CD28-CD80/86 axis
Small EV’s, sometimes termed exosomes, have long been understood to play a variety of roles in tumorigenesis and cancer progression, including extracellular membrane remodeling, angiogenesis, tumor invasion, treatment resistance, and immunosuppression. Yet, only recently have we understood how to modulate these relationships and subsequent tumor ICB response.11 One important study examined PD-L1, a protein that helps keep the body’s immune responses under control, and when it binds with another protein, it keeps T cells from killing cancer cells.
Chen and colleagues found that induced expression of PD-L1 in EVs using IFN-γ, a dimerized soluble cytokine in the type II class of interferons, led to tumor immunosuppression, which, in turn, increased responsiveness to ICB therapy. Yet, treatment options for PD-1/PD-L1 ICB refractory disease are limited, which has led to significant research into additional T-cell immunosuppression pathways.
Indeed, CTLA4 antibodies targeting the immunosuppressive CD80/86 receptor on T-cells have been shown to be effective in treating numerous immunotherapy-sensitive solid tumors. But these therapies have not proven as effective as PD-1/PD-L1 targeted therapies, and recent studies have suggested that the CD 80/86 co-receptor CD28 may be a better target for inhibition.15 Thus, Zhao and colleagues investigated whether EV’s could be utilized to modulate the CD28-CD80/86 axis and increase T-cell cytotoxic activity against colorectal cancer.
In their study, Zhao and colleagues first identified that the CD28-CD80/86 axis was dysregulated on tumor infiltrating T-cells and dendritic cells, though the expression levels were surprisingly invariant between microsatellite stable (MSS) or MSI tumors. Using genetic knockout mice, they found that the absence of CD28 and CD80/86 prevented colorectal cancer xenograft (a tissue graft from a donor of a different species) response to ICB therapy. Then, utilizing a microRNA (miRNA) screen of miRNAs specifically overexpressed in CRC, they identified miR-424 as a negative regulator of the CD28-CD80/86 pathway, suggesting that this miRNA may be at least partially responsible for tumor immunosuppression.
The authors found that tumor-derived EVs are a significant mechanism for delivery of miR-424 to its T-cell and dendritic cell targets. Then, using miR-424 knockout tumors, the authors showed that these tumors generated EVs without miR-424, and that this absence leads to immune-driven tumor suppression (See Figure 1.).
Finally, the authors found that these miR-424 absent EVs could then be administered to a separate xenograft, and lead to significantly improved ICB efficacy against those tumors. Thus, the authors established that miRNA depleted or absent EVs may be a critical adjunct in CRC ICB therapy, leading to rescue of treatment resistance, or the induction of treatment response in the vast majority of CRC that does not respond to ICB therapy.
Conclusions
There have been significant advances in the targeted treatment of therapeutic biomarkers in CRC, especially as related to immunotherapy. However, we are still developing our understanding of tumor characteristics that affect treatment, and the optimal modalities with which they can be paired. While exciting advances are being made in multiple avenues, the results presented by Zhao and colleagues, utilizing modified extracellular vesicles are unique in multiple respects. They represent a mutation agnostic methodology by which CRC may be sensitized to immunotherapy, regardless of molecular subtype or location of metastases. In addition, their study involved the usage of tools that are already in clinical investigation. Given promising results from multiple early phase EV clinical trials, these data may present a novel therapeutic option for a large population of CRC patients for whom treatment options are currently limited.
References
- National Cancer Institute. SEER Cancer Annual Report to the Nation 2021: Overall Cancer Statistics. 2021; https://seer.cancer.gov/report_to_nation/statistics.html. Accessed September 1, 2021.
- National Comprehensive Caner Network, N.C.C. Colon cancer. Version 2. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Updated August 11, 2021.Accessed September 2, 2021.
- Lee, C, et al, Enhancing the landscape of colorectal cancer using targeted deep sequencing. Sci Rep. 2021; 11(1): 8154. doi: 10.1038/s41598-021-87486-3.
- Van Cutsem, E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009; 360(14): 1408-17. doi: 10.1056/NEJMoa0805019.
- Hong D, et al. KRASG12C Inhibition with Sotorasib in advanced solid tumors. N Engl J Med. 2020; 383(13): 1207-1217. doi: 10.1056/NEJMoa1917239.
- Drilon A, et al. Efficacy of Larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018; 378(8): 731-739. doi: 10.1056/NEJMoa1714448.
- Wang H, et al. NTRK fusion positive colorectal cancer as a unique subset of CRC with high tumor mutation burden and microsatellite instability. J Clin Oncol. 2021. 39(15_suppl): 3544-3544.
- Guo Y, et al. Genomic alterations of NTRK, POLE, ERBB2, and microsatellite instability status in chinese patients with colorectal cancer. Oncologist. 2020; 25(11): e1671-e1680. doi: 10.1634/theoncologist.2020-0356.
- Liu J, et al. Immune landscape and prognostic immune-related genes in KRAS-mutant colorectal cancer patients. J Transl Med. 2021; 19(1): 27. doi: 10.1186/s12967-020-02638-9.
- Wang, C., et al. Clinical Response to Immunotherapy Targeting Programmed Cell Death Receptor 1/Programmed Cell Death Ligand 1 in Patients With Treatment-Resistant Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. JAMA Netw Open. 2021. 4(8): e2118416. doi: 10.1001/jamanetworkopen.2021.18416,
- Chen G, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018; 560(7718): 382-386. doi: 10.1038/s41586-018-0392-8.
- Poggio M, et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019. 177(2): 414-427.e13. doi: 10.1016/j.cell.2019.02.016.
- Vermeer, P.D., Exosomal induction of tumor innervation. Cancer Res. 2019; 79(14): 3529-3535. doi: 10.1158/0008-5472.CAN-18-3995.
- Zhao X, et al. Tumor-secreted extracellular vesicles regulate T-cell costimulation and can be manipulated to induce tumor-specific T-cell responses. Gastroenterology. 2021; 161(2): 560-574.e11. doi: 10.1053/j.gastro.2021.04.036.
- Ville S. et al. Co-stimulatory blockade of the CD28/CD80-86/CTLA-4 balance in transplantation: Impact on memory T cells? Front Immunol. 2015; 6: 411. doi: 10.3389/fimmu.2015.00411.
- NCI dictionary of Cancer TERMS. National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-terms. Accessed September 2, 2021.
About the Author

Ajay Prakash, MD, PhD
is an Assistant Professor in the Division of Hematology, Oncology, and Transplantation, Department of Medicine, at the University of Minnesota Medical School.