Minimizing laboratory errors with automation
For a printable version of the August CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the type and rates of errors in the three stages of the laboratory testing process.
2. Describe the reasons for pre-analytical errors and their impact.
3. Describe how pre-analytical efficiency can be improved.
4. Explain how the COVID-19 pandemic has renewed the urgency for automation.
Laboratories play a crucial role in both individual and population-based healthcare — and use various methods to reduce errors, ensure patient safety, and improve quality.
Laboratory testing is often used to confirm initial impressions or rule out differential diagnoses. It is estimated that 70% of all healthcare decisions affecting diagnosis or treatment involve laboratory testing,1 and at least 10% of all diagnoses are not considered final until laboratory testing is complete.2,3 Published data suggest that 24-30% of laboratory errors influence patient care, while actual or potential patient harm occurs in 3-12% of cases.4,5,6
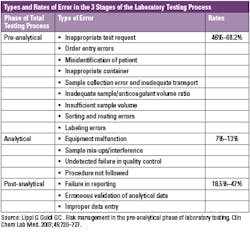
There is clear evidence that most laboratory errors fall outside the analytical phase, and pre- and post-analytical processes are more vulnerable to error.7,8 Pre-analytical errors account for up to 70% of all mistakes made in laboratory diagnostics, most of which arise from problems in patient preparation, sample collection, transportation and preparation for analysis and storage.9
Research shows that, on average, the cost per pre-analytical error in the United States is $208; and without intervention, an average laboratory can expect to incur approximately $180,000 per year in costs related to mislabeling, wrong samples, and insufficient sample volumes. For example, a typical mid-sized hospital laboratory processes around 182,500 tubes per year. Studies show that approximately 0.66% of these tubes will come into the laboratory with pre- or post-analytical errors, and 72% of those tubes will directly contribute to additional costs.10,11 Given the expense, laboratorians must focus their attention on pre-analytical and post-analytical processes,12 as these phases seem to present the greatest potential for quality improvement once reliable strategies have been identified and properly applied.
The error factor
In a one-year study, researchers found that for inpatients, there was a pre-analytical error rate of 1.9%. The variable receiving the highest frequency rating was specimen hemolysis at 1.10%. The error rate was 1.2% for the outpatients, and the variable with the highest frequency rating was insufficient volume for testing.13 Some of the other common pre-analytical errors that are found on average at the rate of 46%–68.2% in laboratories include:14
- Inappropriate test request
- Order entry errors
- Misidentification of patient
- Inappropriate container
- Sample collection error and inadequate transport
- Inadequate sample/anticoagulant volume ratio
- Insufficient sample volume
- Sorting and routing errors
- Labeling errors
The human factor
Health system costs in clinical laboratories are incurred daily due to human error. Indeed, a major impetus for automating clinical laboratories has always been the opportunity it presents to simultaneously reduce cost and improve the quality of operations by decreasing human error.15
Approximately 60% of laboratory technicians are spending their time in the pre-analytical phase: accessioning, sorting, decapping, centrifuging, transporting, etc. Up to 75% of testing errors take place during the pre-analytical phase,16 all of which can lead to reporting delays and errors. This is mainly attributed to the difficulty in achieving standardized procedures for sample collection,17 posing a great challenge for laboratories.
The automation advantage
Overcoming challenges with staffing, fluctuations in testing volumes, improving turnaround time, and reducing errors and costs are all proven benefits that have fueled the adoption of laboratory automation. Today, even labs processing smaller volumes of samples – ranging from one to three million tests per year or as low as 500 samples per day, often enjoy the benefits of lab automation to help address their challenges.18
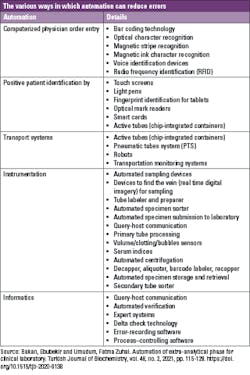
Automation allows for taking over the bulk of many manual ordinary activities (i.e., specimens sorting, loading, centrifugation, decapping, aliquoting, sealing) from humans, thus minimizing substantial differences among persons and from sample to sample.19 Such improved process standardization can yield tangible benefits on the quality of the total testing process, and lower the risk of diagnostic errors, especially those emerging from the manually intensive activities of the pre-analytical phase.20 In addition, automation helps to mitigate bottlenecks that occur during sample preparation by processing samples quicker and more efficiently with less variation than humans.21
Improving pre-analytical efficiency
The aim of introducing automation in the pre-analytical phase is to prevent human error, which is exacerbated by the fact that laboratory workers are currently handling ever-increasing workloads alongside a reduction in personnel, leading to physical and mental exhaustion. Automated robotic workstations effectively reduce the number of laboratory errors that occur in sorting, labeling, and aliquoting specimens, which improves the integrity of those specimens throughout the steps of specimen processing.22 Research shows that effective integration between automation and information management is key to assuring a more sophisticated control of laboratory processes.23 In fact, automation technologies have had a big impact on the proficiency of clinical laboratories. To improve standardization, organization, efficiency, and quality of the total testing process, many manual tasks have now been partially or completely automated by labor-saving instrumentations, and the cost of the investment will possibly return on a long-term basis.24,25
The impact of automation on human resources
Automation plays a key role in addressing staff shortages, while enabling precious and highly skilled human resources to focus on high-value clinical tasks, particularly during the COVID-19 pandemic.18 Even though molecular testing, which is the gold standard for diagnosing COVID-19, is not included in current workflow automation solutions, automating manual processes for routine tests frees up lab resources to do more COVID-19 or other dedicated scientific work.27
Automation solutions also play an important role in improving morale and work quality for laboratory staff. For example, manual labeling of tubes is not only tiring and time-consuming, these routine tasks of labeling, filling, uncapping, and capping of tubes can result in stress injuries in the fingers; in many cases, it leads to carpal tunnel syndrome. Automation can complete these tasks and decrease the risk of repetitive injuries from manual labeling. In addition, using automated solutions for repetitive tasks enables laboratories to lighten the workload on staff, so they can focus on more important high-value functions.28
The impact of automation on laboratory logistics
All laboratories, regardless of their size, can benefit from some level of automation, but implementing an automation system should not be done haphazardly. Rather, the design and implementation of such a system must follow a thorough analysis of a laboratory’s current — and future — testing requirements. Only then will all of the information be available to determine what level of automation is sufficient29 and acceptable within the logistical parameters of the lab (example: space and budget).
The design and layout of a clinical laboratory impacts the operational efficiency, flexibility, and costs of a healthcare organization throughout its operational life.30 Optimal space requirements should be adhered to as to ensure work remains unhindered and employee safety is upheld.31
The instrumentation required to perform the test menu in the laboratory and the degree of automation are the primary drivers of space, which is expensive and a precious commodity in the acute care setting. Therefore, optimization of specimen processing is the central focus within most clinical laboratories. Among the key clinical lab design principles are workflow efficiency for improved specimen processing; flexible and modular space allocation to accommodate robotic instrumentation; and safe, people-centric design to promote healthy work environments.32
As lab work continues to move away from manual bench testing to increasingly more automated processes, open-plan designs provide the flexibility necessary for labs to easily add analyzers or adapt to provide more efficient workflows.33
Automation solutions can offer laboratory managers greater control over their workspace, while allowing flexibility to scale according to test volumes, laboratory size and cost requirements.
COVID-19: a renewed cry for automation
COVID-19 has put the human factor of laboratory technicians to the test. Laboratory staff is facing tremendous pressure resulting from the sheer magnitude of the pandemic, including keeping up with the high volume of tests per day and adhering to COVID-19-related regulations, all while staying safe. With testing volumes higher than ever, laboratory professionals are working extended hours and across multiple departments and laboratories. Post-pandemic, the number of tests needed are predicted to increase, as those who put off annual check-ups, about 41% of U.S. adults according to the CDC,36 will start testing again. In August 2020, an AACC survey found that 58% of laboratories noted staffing as an issue, up from 35% in May,37 and the gap between laboratories finding available, qualified medical laboratory technicians and the demand for that skillset continues to expand. Consequently, the late shift, when fewer people are around, is being assigned to less-experienced technicians. This coupled with the laborious, repetitive nature of lab work leaves additional room for human errors to occur, leading to increased lab operating costs.38
Automation plays a key role in helping to address staff shortages while enabling precious resources to focus on high-value clinical tasks, and this is particularly true during the COVID-19 pandemic. Among the changes that the COVID-19 pandemic has affected is an acceleration in laboratories automating their workflows. The driving force behind increasing automation was the need to increase efficiency as test volumes soared during the peak of the pandemic. Another key component was the need to reallocate staff to tasks that required more critical thinking and a human touch, rather than basic sample preparation that could be done by a robot. Many laboratorians also noted the ongoing shortage of qualified lab technicians and said that automation had helped them make the best use of the techs they have.39
Beyond instruments and equipment, lab leaders should adopt an entirely new mindset when it comes to workflow improvements. Some of the greatest limitations in the lab aren’t technical at all — they’re mental. Resistance to automation and inability to be flexible to new technological demands will almost certainly impede success in future crises. With a critical eye, each area and manual/mechanical process in the lab should be examined for efficiency. Those that prove to be a bottleneck in the process are where automation is best focused.40
Conclusion
There are many advantages to introducing automation during the pre-analytical phase. These advantages include a decrease in repeat specimen collection; reduced specimen volume; secure patient and specimen identification; achievement of effective specimen integrity and preservation; decreased specimen handling, which helps laboratory personnel to avoid blood-based infection; containment of biohazardous materials; avoiding risk of human error; and reduction of the number of test tubes used.41
The sheer magnitude of the pandemic means that laboratory automation technologies are being embraced more than ever for their ability to drastically reduce the bottlenecks in sample prep and process samples at a much faster rate than humans. Although COVID-19 testing and research efforts are currently the main drivers, the need for lab automation has been accelerated in other laboratories, too, as a means of helping them operate more safely and efficiently.42 The facts are clear. Laboratories play a central role in safeguarding the well-being of patients. Automation is a tool that enables laboratorians to perform this role much more efficiently and effectively.
References
- Badrick T. (2013) Evidence-based laboratory medicine. Clin Biochem Rev. 2013 Aug; 34(2): 43–46.
- Peterson MC, Holbrook JH, Von Hales D, et al. Contributions of the history, physical examination, and laboratory investigation in making medical diagnoses. West J Medicine. BMJ 1992;156(2):163–5.
- Wahner-Roedler DL, Chaliki SS, Bauer BA, et al. Who makes the diagnosis? The role of clinical skills and diagnostic test results. J Eval Clin Pract. 2007;13 (3):321-5. doi: 10.1111/j.1365-2753.2006.00691.x.
- Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem. 2007 Jul; 53(7):1338-42. doi: 10.1373/clinchem.2007.088344.
- Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem. 2010 Mar; 47(Pt 2):101-10. doi: 10.1258/acb.2009.009222.
- Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem. 1997 Aug; 43(8 Pt 1):1348-51.
- Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem 2002;48:691-8.
- Astion ML, Shojana KG, Hamil TR, Kim S, Ng VL. Classifying laboratory incident reports to identify problems that jeopardize patient safety. Am J Clin Pathol. 2003;120(1):18-26. doi: 10.1309/8EXC-CM6Y-R1TH-UBAF.
- Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D, et al. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 2011;49:1113–26. doi: 10.1515/CCLM.2011.600.
- Barak M, Jaschek R. A new and effective way for preventing pre-analytical laboratory errors. Clin Chem Lab Med. 2014 Feb;52(2):e5-8. doi: 10.1515/cclm-2013-0597. PMID: 24096442.
- Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem. 2013 Sep;46(13-14):1175-9. doi: 10.1016/j.clinbiochem.2013.06.001. Epub 2013 Jun 14. PMID: 23769816.
- Howanitz PJ. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Pathol Lab Med. 2005;129:1252-61. doi: 10.5858/2005-129-1252-EILMPL.
- Chawla R Goswami B Tayal D et al. Identification of the types of preanalytical errors in the clinical chemistry laboratory: 1-year study at G.B. Pant Hospital. LabMedicine. 2010;41:89–92.
- Lippi G Guidi GC . Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med. 2007;45:720–727. doi: 10.1515/CCLM.2007.167.
- Bissell MG. Information systems and human error in the lab. Clin Leadersh Manag Rev. 2004 Nov-Dec;18(6):349-55. PMID: 15597557.
- Bonini P, Plebani M, Ceriotti F et al. Errors in laboratory medicine. Clin Chem. 2002;48(5):691–698.
- Rana S. No preanalytical errors in laboratory testing: a beneficial aspect for patients. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3477456/. Accessed June 28, 2021.
- Gramz J. The benefits of lab automation facilitate testing for SARS-CoV-2. November 19, 2020. Medical Laboratory Observer. Accessed June 21, 2021. https://www.mlo-online.com/continuing-education/article/21163060/the-benefits-of-lab-automation-to-facilitate-testing-for-sarscov2.
- Seaberg RS, Stallone RO, Statland BE. The role of total laboratory automation in a consolidated laboratory network. Clin Chem. 2000;46:751–6.
- Yeo CP, Ng WY. Automation and productivity in the clinical laboratory: experience of a tertiary healthcare facility. Singapore Med J. 2018;59:597–601.
- Appel, A. How Lab Automation is Supporting COVID-19 Testing and Research. Strategic Directions International. July 14, 2020. https://strategic-directions.com/lab-automation-supporting-covid-19-testing-research. Accessed: March 1, 2021.
- Holman JW Mifflin TE Felder RA Demers LM . Evaluation of an automated preanalytical robotic workstation at two academic health centers. Clin Chem. 2002;48:540–548.
- Plebani M. Laboratory errors: How to improve pre- and post-analytical phases?. Biochem Med. (Zagreb). 2007;17:5-9.
- Zaninotto, M, Plebani, M. The “hospital central laboratory”: automation, integration and clinical usefulness. Clin Chem Lab Med. 2010;48:911–7. https://doi.org/10.1515/cclm.2010.192.
- Dolci, A, Giavarina, D, Pasqualetti, S, Szőke, D, Panteghini, M. Total laboratory automation: do stat tests still matter? Clin Biochem 2017;50:605–11. https://doi.org/10.1016/j.clinbiochem.2017.04.002.
- Addressing the clinical laboratory workforce shortage. The American Society for Clinical Laboratory Services. Aug 2, 2018. https://ascls.org/addressing-the-clinical-laboratory-workforce-shortage/. Accessed June 28, 2021.
- Wolski C. How COVID-19 is driving the need for lab automation. Clpmag.com. Published May 18, 2021. https://clpmag.com/lab-essentials/lab-automation/how-covid-19-is-driving-lab-automation/. Accessed June 21.
- Rupp S. Electronic health reporter. Electronichealthreporter.com. Published April 9, 2018. https://electronichealthreporter.com/the-benefits-of-automation-in-hospital-management/ Accessed June 21, 2021.
- Berman R. Designing and implementing laboratory automation for improved patient safety. Lab Med. 2004;35(1):52-57.
- Shukla CS. Lab work. Hfmmagazine.com. Accessed June 22, 2021. https://www.hfmmagazine.com/articles/1002-lab-work.
- Ezelle J et al. Guidelines on good clinical laboratory practice: bridging operations between research and clinical research laboratories. J Pharm Biomed Anal. 2008;46(1):18–29. doi: 10.1016/j.jpba.2007.10.010.
- Better approaches to clinical laboratory design. Clinicallabmanager.com. https://www.clinicallabmanager.com/lab-design-and-furnishings/better-approaches-to-clinical-laboratory-design-23246. Accessed June 22, 2021.
- Jaeger A. Designing clinical labs. Hfmmagazine.com. https://www.hfmmagazine.com/articles/1703-designing-clinical-labs. Accessed June 22, 2021.
- Phua, J. et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir. Med. 8, 506–517 (2020). doi: 10.1016/S2213-2600(20)30161-2.
- Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 581, 465–469 (2020). doi: 10.1038/s41586-020-2196-x.
- Czeisler MÉ, Marynak K, Clarke KE, et al. Delay or Avoidance of Medical Care Because of COVID-19–Related Concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1250–1257. doi: http://dx.doi.org/10.15585/mmwr.mm6936a4.
- Medical Laboratory Technician Demographics. (2021, April 30). Zippia Career Expert. https://www.zippia.com/medical-laboratory-technician-jobs/demographics/. Accessed July 9, 2021.
- Gallup’s “State of the Global Workplace” Report, 2017.
- Ketchum K. Spurred on by COVID-19 pandemic, laboratories accelerate automation efforts. 360Dx.com. Published June 2, 2021. https://www.360dx.com/clinical-lab-management/spurred-covid-19-pandemic-laboratories-accelerate-automation-efforts. Accessed June 22, 2021.
- Schmidt, C. Lab automation in a post-COVID world: 5 questions to ask and answer. Labmanager.com. Published April 30, 2021. https://www.labmanager.com/big-picture/lab-management-in-crisis/lab-automation-in-a-post-covid-world-5-questions-to-ask-and-answer-25772. Accessed June 21, 2021.
- Rachna Agarwal, MD, Quality-improvement measures as effective ways of preventing laboratory errors, Laboratory Medicine, Volume 45, Issue 2, May 2014, Pages e80–e88. doi.org/10.1309/LMD0YIFPTOWZONAD.
- Appel A. How lab automation is supporting COVID-19 testing and research - strategic directions. Strategic-directions.com. Published July 14, 2020. https://strategic-directions.com/lab-automation-supporting-covid-19-testing-research/. Accessed June 21, 2021.
About the Author

Jacqui Reithel, MBA, MT (ASCP)
is a Senior Manager in Process Consulting for Beckman Coulter.