The benefits of lab automation to facilitate testing for SARS-CoV-2
Earning CEUs
For a printable version of the December CE article and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the challenges in the lab that have been exacerbated by COVID-19
2. Describe the role of serology testing during the COVID-19 pandemic
3. Describe the challenges that automation in the lab addresses
4. Discuss the key capabilities lab automation provides during the pre-analytical, post-analytical and data management phases of lab testing
Fear, anxiety, uncertainty, and worldwide economic disaster have all been factors stemming from the COVID-19 pandemic. Disruption is the new normal to daily life with very few areas of our personal or professional lives not impacted in some way. Although measures to “flatten the curve” and reduce the number of new COVID-19 cases from one day to the next have helped to prevent healthcare systems from becoming overwhelmed, the pandemic has exacerbated many of the existing challenges laboratories commonly face and also has introduced a number of new ones to overcome.
Test menu expansion, fluctuations in testing volumes, consistent supply of consumables, access to personal protective equipment and ongoing changes in management to a new way of working were all new challenges to which laboratories have had to adapt throughout the pandemic. Automation and leveraging technology and innovation within the lab can play a key role in helping laboratories minimize disruption and overcome the challenges of testing for COVID-19.
Testing for a new pandemic
As of early October, the number of confirmed COVID-19 infections surpassed more than 35 million confirmed cases and accounted for more than 1 million deaths worldwide.1 Within the United States, there have been more than 7,679,908 cases, and 215,039 deaths since the first domestic case of COVID-19 was reported on January 21, 2020. Diagnostic testing was quickly identified as a critical component to battle the pandemic and multiple initiatives were put in place to help increase the availability of testing. Some of these included accelerating technology availability through emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA), expanded testing capacities and enabling federally funded surge testing in partnership with communities experiencing outbreaks.2
The need for laboratories to expand testing services to support viral testing for SARS-CoV-2 diagnosis was one of the earliest new challenges introduced. Labs were forced to either expand their menu of testing services or identify an alternative process to outsource viral testing to other laboratories. Diagnostic testing was quickly made possible and testing rates increased drastically, growing from 20,000 tests per day in April to more than 1,100,000 tests per day in early October.3
Testing capabilities were then further expanded with serology-based antibody testing. These tests detect the presence of antibodies in the blood based on the body’s immune response to the COVID-19 infection. Laboratories prepared for antibody testing to ramp up in mass numbers, anticipating demand as results would provide greater detail and data to help safely reopen communities. Meanwhile, antibody testing evolved in sophistication from simple qualitative positive/negative results to semi-quantitative numerical measurements gauging the specific level of IgG antibodies within a patient’s blood sample.4
The expansive rollout of such tests, however, was not immediately realized. The pandemic was still in its infancy and, as a result, confirmatory diagnostic tests remained in greater demand. While antibody testing is not deemed suitable for diagnosis of COVID-19, studies to help determine the appropriate use of serology testing for SARS-CoV-2 are underway. Potential benefits may include helping healthcare professionals identify individuals with an immune response to SARS-CoV-2, as well as blood donor candidates to enable convalescent plasma therapy for patients seriously ill from COVID-19.5
Other key benefits could include enabling clinicians to assess a patient’s natural immunity acquired through viral infection, as well as helping to determine the potential effectiveness of vaccines. To help make this possible, IVD vendors and public health institutions – like the Centers for Disease Control and Prevention (CDC) within the United States and the JRC (Joint Research Centre) of the European Commission – are working together to establish a standardized process related to SARS-CoV-2 assays that will enable clinicians to track their patients’ antibody concentrations, regardless of the test method or manufacturer used.6
New crisis, same key issues
A recent health-crisis readiness survey conducted by consulting and lab optimization firm Accumen assessed the level of preparedness hospitals and health systems had with respect to the COVID-19 pandemic. The survey included responses from 242 health system leaders with representation ranging from small hospitals to large integrated systems and from rural areas to large cities across the United States.
When asked to identify the key challenges hospital labs are facing, staffing (26 percent) and turnaround time (23 percent) were identified as the top priorities, with information technology/laboratory information system resources (17 percent) and supply chain (17 percent) also receiving high response rates as priorities.7
Staffing challenges, including employee turnover and the inability to hire qualified staff, remain long-term chronic problems facing the laboratory. The Bureau of Labor Statistics (BLS) projects a nationwide increase in the demand for medical and clinical laboratory technologists of 13 percent between 2016 and 2026. The Human Resources and Service Administration (HSRA), within the Department of Health and Human Services (HHS), projects a growth in demand of nearly double that amount, or 22 percent, between 2012 and 2025. In addition, vacancy rates remain high, averaging 7.2 percent across the nation.8 Interject the COVID-19 situation, with the potential to reduce availability of lab staff due to personal or health reasons, together with the need to expand services for SARS-CoV-2 testing, and the age-old problem of the lab needing to do more with less is compounded.
Automation plays a key role in helping to address staff shortages while enabling precious resources to focus on high value, clinical tasks, and this is particularly true during the COVID-19 pandemic.
Turnaround time (TAT) may be king of laboratory metrics when it comes to monitoring key performance indicators, with some medical professionals seeing TAT as something almost as important as the quality of test results themselves.9 Establishing the logistics needed to enable new testing services for COVID-19 – coupled with the lead time to collect and process samples – and the extreme surge in testing volumes has led to much attention and consternation on the amount of time it takes for patients to receive results from testing for COVID-19. Turnaround times for COVID-19 testing can vary significantly, depending on the type of test being performed, the analyzer or device being used, and the logistics involved in ordering the tests. The processes for collecting and receiving the sample, performing the test and reporting the results also impact turnaround times.
TAT for the actual processing of tests for COVID-19 varies greatly depending upon the type of testing being performed, and can range from as little as 10 minutes for industry-leading serology-based antibody tests, 15 to 30 minutes for antigen-based POC testing, 15 to 45 minutes for POC-based molecular testing and up to 7 hours for reverse transcriptase (RT) polymerase chain reaction (PCR) testing.10 For serology-based testing, fully automated laboratories are better positioned to optimize TAT by eliminating the need for human intervention between phases and leveraging the added benefits of full transparency and control over where the samples are throughout the process.
For many labs where information technology or LIS resources already pose a challenge, the incremental impact of COVID-19 adds fuel to the fire. With many labs quickly expanding menus to include additional testing for both viral- and serology-based antibody testing, the demand for what their existing lab IT solutions can offer and the service and support needed to make it happen come into play. The challenges can vary with some labs positioned to simply add new tests to existing testing platforms, while other labs face the need to procure, implement, interface and commission new analyzers and support data workflows. And then beyond these common scenarios, comes the incremental challenge for all COVID-19 diagnostic and screening test sites to report results to the appropriate state or local public health department daily and within 24 hours of test completion.11
Leveraging automation in the wake of COVID-19
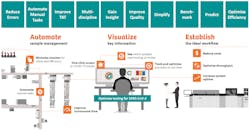
Adoption of laboratory automation has advanced significantly since the first total laboratory automation solution was implemented in North America back in 1996.12 Overcoming challenges with staffing, fluctuations in testing volumes, improving TAT, and reducing errors and costs are all proven benefits that have fueled adoption of laboratory automation. Today, even labs processing smaller volumes of samples – ranging from 1 million to 3 million tests per year or as low as 500 samples per day – often leverage the benefits of lab automation to help address their challenges. (See figure 1)
The good news for many labs already equipped with automation technology and innovation is the built-in capabilities and scalability they may have available to help overcome many of the COVID-19-related challenges. This includes physical laboratory automation systems used to automate and reduce the number of process steps and manual touchpoints and then to expedite the physical handling, sorting and distribution of samples. It also includes software-based lab IT solutions to automate and optimize workflows associated with generating patient and Quality Control (QC) results and the visualization and management of all the information they need. Those laboratories already leveraging the benefits of total lab automation to perform multidisciplinary testing have been well equipped to not only accommodate the need for serology-based antibody testing but also the incremental volume of coagulation, hematology and immunochemistry testing necessary for patients impacted by COVID-19.
When expanding lab services to include serology testing for COVID-19, there are several key workflow considerations that need to be evaluated for the lab to establish an ideal workflow. Which instrument(s) will work best for antibody testing? What is the expected testing volume and how will it fluctuate over time? Will other tests need to be processed on the same sample and which ones should be processed first? Should I integrate testing onto my automation system or manage it directly on a stand-alone instrument? Will testing be performed on demand or will COVID-19 samples be batch tested? Is there any special post-processing sorting or storage criteria?
All of these questions require careful evaluation to establish the right strategy and implement the right workflow. Ensuring that the solution provider has the expertise, consulting capabilities and tools needed to help you design, implement and operate the right automation solution for your laboratory is key.
Optimizing the pre-analytical phase
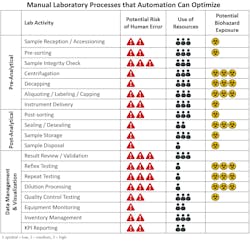
The pre-analytical phase of testing can be resource-intensive and highly prone to error. A study done to evaluate the frequency and types of errors that occur in laboratory medicine provided evidence that most errors occur during the pre-analytical phase of testing and can include anywhere from 31 percent up to 75 percent of total laboratory errors.13 Some of the most common types of pre-analytical errors include hemolyzed samples, insufficient sample volumes, incorrect or mislabeled samples and clotted samples. For COVID-19 patients at risk of complications, and whose treatment is often dependent upon sometimes minor differentiations across comparative test results, erroneous error can gravely impact timely treatment.
Lab automation can lead to dramatic improvements in staffing, TAT, and error reduction. It can even reduce the risk of staff exposure to biohazardous materials. (See figure 2) Some of the key capabilities that lab automation provides to support pre-analytical activities include:
- Flexible sample loading options, which can accommodate different scenarios, with multiple entry points and for different volumes of samples, including individual sample loading, rack loading and automated bulk loading for large volumes of samples. The benefits include centralized loading for multiple tube sizes, multiple sample types, capped and uncapped tubes, spun and unspun samples, STAT and routine samples. This reduces effort for laboratory staff by eliminating the need to presort and standardize samples.
- Priority routing, presorting and batch-testing management, which enable labs to manage how and in what sequence samples are processed for COVID-19. Options include the ability to specify and automate which instruments are used and which tests get processed first, as well as the ability to presort samples and automate batch-testing processes.
- Sample inspection modules, which can be used to quickly and automatically evaluate sample integrity and identify potential errors due to incorrectly capped or uncapped tubes, mislabeled or unreadable samples, incorrect sample types or tube types, hemolyzed samples and samples with insufficient volume.
- Online centrifugation, which saves time, reduces the need for manual handling and can reduce the risk of exposure to biohazardous materials through offline centrifugation. Additional benefits include automated load balancing to accommodate multiple sample types, configurable wait times, spin cycle times and rotation speeds, temperature setting and STAT sample priority unloading.
- Decapper modules, which increase productivity and safety of lab staff while decreasing risk of sample contamination for better quality results.
- Aliquoting and recapping, which automates sample handling for send-outs and special storage scenarios (long-term and frozen) while reducing exposure to biohazardous materials.
Streamlining the post-analytical phase
The same study showed that errors occurring during the post-analytical phase of testing also have a significant impact and contributed to anywhere from 9 percent up to 30.8 percent of total laboratory errors.13 Similar to the pre-analytical phase, lab automation offers a number of specific capabilities that can help address challenges within the post-analytical phase of testing including:
- Post-processing sorting and output modules, which enable quick operator access to “problem” samples and sorted tubes and provides temporary storage leading to fast, automated sample retrieval and analyzer delivery for reflex testing, repeat testing, add-on tests, and other custom scenarios that may require sending samples to output racks or sorting lanes. These capabilities may be especially beneficial for labs processing multiple tests on SARS-CoV-2 samples where add-on and reflex testing may be necessary.
- Refrigerated storage and de-sealing, which enables all of the benefits of automated sample retrieval and routing for reflex and repeat testing for temperature-controlled samples stored for longer periods of time. In addition, automated sample disposal based on test-life viability enables samples to be automatically discarded while reducing the risk of exposure to biohazardous materials.
The need for diagnostic software
The ability to visualize key information and understand how the lab is performing is critical. Identifying where attention is needed and enabling lab staff to quickly take the appropriate action to resolve issues is key for any laboratory. To accommodate this need, many labs leverage middleware to complement and supplement the capabilities of their laboratory information system. These often include solutions for advanced data management, process management, inventory management and analytics that include tight integration with the analyzers and lab automation systems and play a key role in simplifying operations and accommodating the specific workflow needs of the lab. Specific functions include:
- Advanced data management, which enables the lab to standardize operations for patient and QC result management while maximizing staff efficiency, reducing errors, improving TAT and simplifying the management of daily QC. It includes key capabilities like dashboard visualization of testing status with alerting capabilities to create awareness and drive action. It enables the efficient review of patient results through review by exception, ensuring lab staff focus on the results that need attention and skip the ones that do not. It enables the efficient, real-time monitoring of quality control with the ability to quickly identify, troubleshoot and resolve problems. And it often includes clinical decision support capabilities central to the needs of the laboratory like panic value monitoring and algorithms to support specific testing protocols.
- Process management, which provides centralized oversight and control of the analyzers and automation systems used within the lab and across multiple sites. It enables real-time monitoring and alerting of system issues and on-board consumable inventory status, enabling labs to maximize system uptime and minimize unplanned downtime. It also enables remote view and control of the analyzers and helps avoid the need for staff to walk around the laboratory to physically inspect each system.
- Analytics, which play a crucial role in empowering laboratories to assess performance, identify inefficiencies and determine the root cause of problems. It enables the lab to quickly and easily benchmark performance and drive continuous improvement with the ability to monitor common KPIs through use of reports for TAT, TAT exceptions, throughput, reagent efficiency, automation utilization, auto-validation rates, hemolysis, problem samples and other key metrics important to the laboratory.
- Inventory management, which helps labs reduce cost and improve efficiency by automating many of the manual steps involved with ordering, receiving, utilization and reordering of consumable materials needed to operate their lab.
On the IT side, there are additional needs from an information management perspective when expanding lab services to include serology testing for COVID-19. Labs need to be able to quickly identify how many and which samples are ready for SARS-CoV-2 testing. Which samples resulted for SARS-CoV-2 need review? How many and which samples were tested for SARS-CoV-2? How many and which samples were positive for SARS-CoV-2? All of these can be efficiently managed through the advanced capabilities of diagnostic software.
Conclusion
The impact of the COVID-19 pandemic exacerbated many of the existing challenges labs face with staffing shortages, improving TAT and reducing diagnostic testing errors. The need to once again do more with less within the laboratory is felt worldwide and the trend will continue based on the future availability of lab resources, the need to reduce costs, the aging population and the increasing need for laboratory testing. Automation plays a key role in helping laboratories maximize efficiency, simplify operations and establish a scalable and sustainable approach to minimize disruption to the services they provide and patients they serve.
The demand for serology-based antibody testing will likely increase as clinicians begin to assess patient immunity once vaccines become available. The ability for lab automation and diagnostic IT to accommodate the specific workflow needs of the laboratory will be extremely important to help labs overcome the challenges of testing during this pandemic and future unknown challenges we may face.
References
- Worldometer. COVID-19 Coronavirus pandemic. https://www.worldometers.info/coronavirus/. Accessed October 6, 2020.
- Department of Health and Human Services. HHS details multiple COVID-19 testing statistics as national test volume surges. https://www.hhs.gov/about/news/2020/07/31/hhs-details-multiple-covid-19-testing-statistics-as-national-test-volume-surges.html. Accessed October 6, 2020.
- Johns Hopkins University School of Medicine. Coronavirus Resource Center. Daily state by state testing Trends. https://coronavirus.jhu.edu/testing/individual-states. Accessed October 6, 2020.
- Siemens Healthineers to collaborate with CDC to standardize SARS-CoV-2 assays. Medical Laboratory Observer. https://www.mlo-online.com/disease/infectious-disease/article/21154694/siemens-healthineers-to-collaborate-with-cdc-to-standardize-sarscov2-assays. September 17, 2020. Accessed October 5, 2020.
- U.S. Food & Drug Administration. EUA authorized serology test performance. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance. Accessed October 30, 2020.
- Centers for Disease Control and Prevention. Serology testing for COVID-19 at CDC. https://www.cdc.gov/coronavirus/2019-ncov/lab/serology-testing.html Accessed October 6, 2020.
- National Health System Crisis Readiness Report 2020 Edition. Accumen. August 27, 2020. https://ACCUMEN.com/crisis-report-2020. Accessed October 6, 2020.
- The American Society for Clinical Laboratory Services. Addressing the Clinical Laboratory Workforce Shortage. Aug 2, 2018. http://www.ascls.org/images/publications/Clinical_Laboratory_Workforce_FINAL_20180824.pdf.
- American Association of Clinical Chemistry. What does turnaround time say about your lab. https://www.aacc.org/cln/articles/2014/july/turnaround-time. Accessed October 6, 2020.
- Los Angeles County of Public Health. Coronavirus Disease 2019 General Testing Guidelines. http://publichealth.lacounty.gov/acd/nCorona2019/test.htm. Accessed October 6, 2020.
- Centers for Disease Control and Prevention. How to report COVID-19 laboratory data. https://www.cdc.gov/coronavirus/2019-ncov/lab/reporting-lab-data.html Accessed October 7, 2020.
- Felder RA. Automation: survival tools for the hospital laboratory. Presentation for the Second International Bayer Diagnostics Laboratory Testing Symposium. New York, July 17, 1998.
- Bonini P, Plebani M, Ceriotti F et al. Errors in laboratory medicine. Clin Chem. 2002;48:691–698.
About the Author

Jamie Gramz, BSE, MBA
is head of Digital Applications for Laboratory Diagnostics at Siemens-Healthineers. He has more than 20 years of experience with automation and IT workflow solutions and holds a BSE in chemical engineering and an executive MBA.