Methods of testing platelet count and function
Earning CEUs
For a printable version of the October CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
Passing scores of 70 percent or higher are eligible for 1 contact hour of P.A.C.E. credit.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1) Describe the platelet activation, adhesion, and aggregation process
2) Recall some inherited platelet disorders
3) Describe the types of antiplatelet therapies
4) Discuss the pros and cons of various lab tests to assess platelet count and function
Platelets are powerful and versatile bio-machines serving to mediate clotting in plasma, constantly surveilling the circulatory system for blood loss, acting as a first-line defense to plug holes and stop leaks like a plumber. In addition, platelets also play critical roles in pathogenesis of coronary and thrombotic disorders including strokes, heart attacks, and venous thromboembolism (VTE).
Platelets past and present
In mammals, platelets are anucleate, small circulating, and abundant cells discovered first by Max Schultze in the 1860s but described in greater depth during the 1880s by Italian pathologist Giulio Bizzozero. Platelets were initially described at that time as “discoid corpuscles” containing granules, and distinct from erythrocytes or leukocytes. Initially, scientists referred to them as “piastrine” in Italian, “blutplättchen” in German, and “petites plaques” in French, but later English publications referred to the circulating cell fragments as “platelets.”
Platelets develop from large bone marrow cells called megakaryocytes. Following fragmentation, more than 1,000 platelets per megakaryocyte are released. Lower vertebrates including reptiles, amphibians, fish, and birds carry a similar cell in their blood called thrombocytes, but unlike platelets, thrombocytes have nuclei.
Normal platelet counts range between 150,000 – 400,000 per microliter. Low platelet count, or thrombocytopenia, can lead to bleeding symptoms, while high platelet counts, or thrombocytosis can lead to arterial or venous thrombosis. Platelet counts within the normal range are critical to maintain blood fluidity, together with a functional coagulation pathway, but functional deficiencies of platelets can also occur, called thrombasthenia. Several acquired and hereditary disorders can reduce the platelet count, including pregnancy, deficiencies of vitamin B12 or folic acid, leukemia, systemic lupus erythematosus, heparin-induced thrombocytopenia (HIT) and thrombotic thrombocytopenic purpura (TTP), among several others.
Patients with bacterial or viral sepsis and the resulting disseminated intravascular coagulation (DIC) also show moderate to severely lower than normal platelet counts, with platelet consumption resulting from the coagulation activation process.1 Similarly, patients with SARS-CoV-2 infection and confirmed COVID-19 illness display mild to severe thrombocytopenia, depending on the course of the illness. In a study from Wuhan, China, of 1,476 patients with COVID-19, 20.7 percent had thrombocytopenia overall, and thrombocytopenia was more likely in non-survivors compared to survivors. Of the patients with thrombocytopenia, nadir, or trough platelet count of 0-50,000 per microliter was more likely associated with mortality outcome compared to patients with higher platelet counts.2
Platelet transfusions are given in patients with low platelet counts (< 50,000 per microliter), such as cancer patients receiving chemotherapy or other patients with low platelet count or platelet dysfunction. However, platelet transfusion is not usually required to prevent bleeding unless the platelet count is < 10,000 per microliter.3
Platelet function and activation process
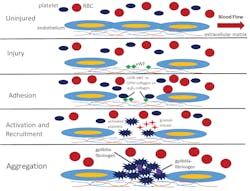
Describing further how platelets function during the activation process, and support hemostasis response to injury, as shown in Figure 1, in an uninjured basal state, platelets are normally in the fluid phase circulating in the blood stream along with erythrocytes, or red blood cells (RBCs), and leukocytes. If tissue injury happens, or if inflammatory processes disturb the endothelial layer, the extracellular matrix containing collagen underlying the endothelial layer will be exposed to the blood flow. The result is binding of von Willebrand Factor (vWF) from the plasma, which then mediates adhesion with platelets flowing in the blood via the GPIb-V-IX-vWF complex. In addition, other platelet receptors mediate adhesion to the collagen in the extracellular matrix, including the GPVI-collagen or α2β1-collagen complexes.
The last step in platelet activation is aggregation, with the gpIIb/IIIa-fibrinogen complexes helping to cement interactions between the activated platelets within the growing plug. Immediately following the aforementioned steps, and simultaneously during the growth of the platelet plug, the plasmatic, or secondary hemostasis system will start forming the fibrin meshwork and compress the platelet plug. Importantly, the platelet surface serves as an anchor for promotion of the plasmatic coagulation processes in vivo, with both the primary and secondary hemostasis processes working together to arrest blood loss. Platelet microparticles or microvesicles shed from activated platelets and megakaryocytes serve to promote coagulation and potential thrombotic effects systemically.
Inherited platelet disorders
The main platelet agonists, or substances producing a physiologic response when bound to certain receptors include TxA2, along with collagen, thrombin, ADP, and others, resulting in activation, shape changes prior to activation, granule secretion, and aggregation. Aggregation will be deficient when abnormalities of the gpIIb/IIIa complex are present, if there are fibrinogen abnormalities, or if any prior step could not proceed fully. The agonists listed are linked to specific receptors, and when the agonist-receptor combination is absent due to a genetic disorder, or targeted by pharmacologic therapies, platelet activation is downregulated along with any linked downstream pathologic effects.
In patients with one of the many known inherited platelet disorders, deficiencies of one of the previously mentioned agonist-receptor combinations may be present, resulting in bleeding symptoms of varying severity. For example, deficiency of the GPIb complex on the platelet surface is found in patients with Bernard-Soulier syndrome, resulting in giant platelets and lack of binding to subendothelium under high shear stress of normal blood circulation.
Other inherited platelet disorders include 1) Glanzmann thrombasthenia, a lack of gpIIb/IIIa receptor leading to aggregation failure, 2) platelet type von Willebrand Disease, resulting from GPIb mutations and a lack of binding to vWF, 3) ADP receptor deficiency, in which the anti-P2Y12 receptor is missing from the surface, leading to a lack of response to the ADP stimulus, or 4) deficiency of one of the collagen receptors GPVI or α2β1, leading to loss of platelet adhesion. Last, various platelet granule disorders have been observed, in which a reduction of dense or α-granules results in loss of platelet communication and recruitment.5
Antiplatelet therapies
Platelet inhibition with one or more antiplatelet medications is required for patients at risk of arterial thrombosis when patients have acute coronary syndrome (ACS), peripheral artery disease (PAD), heart disease, when undergoing percutaneous coronary intervention (PCI) or angioplasty with stent, or stroke prevention following initial occurrence. Several classes of drugs can provide sufficient platelet inhibition in cases of patient risk, including aspirin, targeting the TxA2 pathway, clopidogrel (Plavix), prasugrel (Effient), and other anti-P2Y12 drugs targeting the ADP pathway, along with abciximab (ReoPro) and other anti-gpIIb/IIIa agents targeting the platelet clumping response.6
Antiplatelet therapies can be confused with anticoagulant, or blood thinning therapy, which targets the secondary hemostasis pathway through inhibition of factor Xa (FXa) or thrombin to prevent or treat venous thromboembolism (VTE). Accordingly, inhibition takes place directly on FXa or thrombin, using small molecule inhibitors such as apixaban or rivaroxaban, or with the help of antithrombin in the case of the unfractionated heparin (UFH) and other related heparinoids.
Assessing platelet count and function
To assess suspected inherited platelet disorders, or to assess effectiveness of antiplatelet therapies, various laboratory methods help clinicians diagnose the underlying disorder and monitor therapies. In the case of inherited disorders, clinicians start with an evaluation of the patient’s personal and family history, using the history and physical examination, including assessment of bleeding severity and documenting other potential symptoms related to platelet granule or collagen deficiency, including loss of renal and cardiac function, hearing loss, or facial dysmorphism.7
First line laboratory testing in the context of primary hemostasis testing and identification of potential inherited or acquired platelet disorders involves platelet count determination, commonly performed in a complete blood count (CBC) measurement on an automated hematology analyzer. The hematology analyzer will also give the mean platelet volume (MPV), with high MPV indicating potential platelet overproduction, or providing a warning sign of cancer presence, but also associated with hyperthyroidism, heart disease, diabetes, vitamin D deficiency, stroke, atrial fibrillation, or high blood pressure.
Although hematology studies provide rapid and precise information on platelet count and MPV, there is no information about platelet function, and accuracy may be decreased at low platelet levels. Microscopy performed simultaneously will assist with identification of morphological issues with platelets or other blood cells.
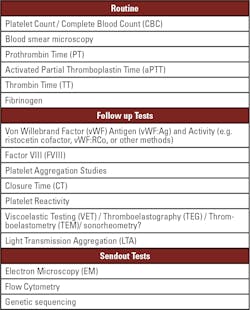
Other routine screening tests include coagulation tests prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, and thrombin time (TT), as shown in Table 1. The coagulation tests allow for ruling out coagulation factor deficiencies, including fibrinogen levels or function. Von Willebrand Factor (vWF) antigen and activity testing provides information about vWF levels or function linked to platelet activity. If vWF antigen levels are decreased, factor VIII (FVIII) levels are often simultaneously decreased since vWF and FVIII are linked together in the plasma to enhance stability and half-life of both proteins. The coagulation tests will commonly be performed on an automated coagulation analyzer, but lack information about platelet function, so coagulation parameters may all be normal in patients with platelet disorders.
Depending on the results of the screening tests, various follow-up tests will be performed depending on equipment available and local practices. Closure time (CT) under high-shear stress performed on a PFA-100 is widely available and assists with identification of platelet or vWF issues, or reflecting anticoagulant drug presence, but the assay is relatively inflexible and sensitive to platelet count and hematocrit variation. Viscoelastic tests (VET) – including thromboelastography (TEG), rotational thromboelastometry (ROTEM), and SEER (Sonic Estimation of Elasticity via Resonance) sonorheometry – allow for investigation of platelet function. VET platforms have the advantage of being available in various clinical settings, including not only central laboratories but also at the point of care.
Various VET methods assess whole blood clotting, providing information on the platelet contribution to clot stiffness and platelet dysfunction, and assist with platelet function analysis along with guiding transfusion during surgeries or emergency procedures. Further, SEER sonorheometry is the only viscoelastic test that directly measures and reports quantification of the platelet contribution to clot stiffness.8 However, VET platforms are not sufficiently sensitive to all functional platelet deficiencies, requiring use of additional tests for a complete diagnosis.
Confirmatory methods for platelet function analysis
Light transmission aggregation (LTA) using platelet rich plasma (PRP) tracks change in light transmission through a sample after stimulation with a panel of various agonists. LTA is the cornerstone and gold standard for platelet function investigation due to the wide panel of agonists used and sensitivity to all inherited and acquired disorders. Disadvantages of LTA include poor standardization, non-automated techniques, and stringent requirements for sample processing and platelet count. Briefly, the mixture of PRP and the selected agonist is stirred to ensure adequate mixing and collision of platelets in the sample, while also preventing sedimentation of platelets. Aggregation is initiated by the addition of an agonist, including ADP for analysis of the P2Y12 and P2Y1 pathways, arachidonic acid and epinephrine for investigation of the TxA2 pathway, collagen for activation of the GPVI-collagen or α2β1 receptors, thrombin receptor activation peptide-6 (TRAP-6) for analysis of the thrombin pathway, and ristocetin to induce potential vWF binding to platelets in the sample.
The agonist added to the sample results in LT increase, and a normal result is obtained if LT reaches the 100 percent level set with the platelet poor plasma (PPP) control. Further analysis of the shape and kinetics of the aggregation curve gives additional information on granule secretion and shape changes indicating various deficiencies. Guidelines from the Clinical Laboratory Standards Institute (CLSI) and the American Journal of Clinical Pathology are available for laboratories that want to implement LTA.9,10
Performing further send out tests for suspected primary hemostasis and platelet disorders is critical to determine the exact diagnosis and tailor treatment accordingly. To assess potential granule deficiencies or problems with other internal structures, scanning electron microscopy (SEM) or transmission electron microscopy (TEM) enables highly sensitive analysis of internal issues, but the equipment and expertise needed are not widely available, and the procedures are time consuming.
As part of the confirmatory process, other send out tests are performed, including flow cytometry using specific antibodies on a small sample size to analyze the number and function of the various activation markers on the platelet surface, along with response to various agonists, even when the platelet count is low. Finally, genetic sequencing procedures will determine the exact mutation responsible for the disorder, giving the clinician sufficient information to manage the patients on a long-term basis.
With the vital contribution of platelets to the overall hemostasis process, and their involvement in many inherited and acquired disease states, it is important that laboratories and clinicians recognize abnormal screening tests indicating potential platelet issues requiring further investigation. Although the method has inherent challenges, LTA occupies a considerable place in platelet disorder diagnosis and monitoring, ensuring placement in laboratory medicine now and in the future.
References
- Levi M, Scully M. How I treat disseminated intravascular coagulation. Blood. 2018 Feb 22;131(8):845-854.
- Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020 Jun;18(6):1469-1472.
- Blumberg N, Heal JM, Phillips GL. Platelet transfusions: trigger, dose, benefits, and risks. F1000 Med Rep. 2010; 2:5.
- Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153-162.
- Handin RI. Inherited Platelet Disorders. Hematology Am Soc Hematol Educ Program 2005; 2005 (1): 396–402.
- Thomas MR, Storey RF. The future of P2Y12 receptor antagonists. Platelets. 2015;26(5):392-8.
- Gresele P, Harrison P, Gachet C, Hayward CPM, Kenny D, Mezzano D, et al. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J. Thromb Haemost 2015; 13 (2): 314 -322.
- Ranucci M, Baryshnikova E. Sensitivity of viscoelastic tests to platelet function. J Clin Med 2020; 9 (189): 1-9.
- Christie DJ, Avari T, Carrington LR, Cohen E, DeBiase BA, Harrison P, Kickler TS, Kottke-Marchant K, Ledford-Kraemer M, Rand ML, Schmaier AH, McCabe White M. Platelet function testing by aggregometry: approved guidelines, H58-A. Clinical and Laboratory Standards Institute: Wayne, PA, USA 2008; 28 (31): 1-45.
- Hayward CPM, Moffat KA, Raby A, Israels S, Plumhoff E, Flynn G, et al. Development of North American consensus guidelines for medical laboratories that perform and interpret platelet function testing using light transmission aggregometry. Am J Clin Pathol 2010; 134: 955-963.
About the Author

Paul Riley, PhD, MBA
serves as Scientific Business Development Manager at Diagnostica Stago, Inc. Paul earned a PhD in biochemistry from Temple University in 2006, with the subject of the dissertation regarding the function of coagulation factor XI. He also did postdoctoral training in a related area before becoming a product manager and scientific affairs specialist in 2009. During his time at Stago, Paul also completed an MBA degree program at Cornell University in 2014. He has spoken to dozens of medical technologist, clinical laboratory scientist, pharmacist, and clinician audiences about various topics within hemostasis and coagulation.