Earning CEUs
For a printable version of the June CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Recall the cause and symptoms of COVID-19 and how severe cases relate to the cytokine storm syndrome (CSS).
2. Discuss normal and abnormal immunologic responses to infection as seen in COVID-19 and CSS.
3. Describe the biological processes that occur in severe cases of COVID-19 and their prognoses.
4. Discuss IL-6 as a CSS biomarker and predictive value for COVID-19 severity.
Introduction
“And the plague gathered strength as it was transmitted from the sick to the healthy through normal intercourse just as fire catches on to any dry or greasy object placed too close to it.”
— Giovanni Boccaccio, Decameron, 1353
While Boccaccio wrote of the Black Death that decimated the population of Europe nearly a millennium ago, his words can easily apply to today’s COVID-19 pandemic plaguing nearly every country in the world. Numbers change daily, but as of this writing in early-May, confirmed COVID-19 infections have surpassed 3,776,454 worldwide,1 accounting for over 261,168 deaths. Of the 1,535,742 closed cases with known outcome, 83 percent of infected individuals have recovered or been discharged from the hospital and 17 percent have succumbed to this modern scourge. However, over 2.2 million cases have yet to resolve, and new infections are reported daily.1
When taking into consideration the total number of cases, both resolved and unresolved, the overall mortality estimated by the World Health Organization (WHO) on March 3 was calculated at ~3.4 percent,2 although this could be lower or higher, depending on local infection prevalence. While this mortality rate is not as severe as either of the two previous coronavirus epidemics (SARS in 2003: 10 percent; MERS in 2009: ~34 percent), mortality is still 52–117 times greater than estimated for annual outbreaks of influenza (0.029–0.065 percent).2 Worse yet, because SARS-CoV-2—the virus responsible for COVID-19—is a novel virus for which there is no widespread immunity, its transmissibility is high, with each infected individual expected to infect ~2–2.5 others if no attempts are made to mitigate spread, such as social distancing.3 In part, this high rate is due to the large number of individuals who are asymptomatic or have only mild symptoms and are not aware they are spreading the illness. Active virus appears to be transmissible several days before symptoms manifest, and individuals might remain contagious for as long as two to four weeks after infection.
Fortunately, ~80 percent of cases are mild, but up to 20 percent of individuals require hospitalization3 for serious or severe symptoms that include mild to marked hypoxia, and ~5 percent of all cases progress to critical symptoms, presenting with severe hypoxia and respiratory distress characteristic of acute respiratory distress syndrome (ARDS) that can rapidly degrade to septic shock and multiple organ dysfunction (MOD) within 12-24 hours of hospital admission. These cases require critical care measures, including the use of vasopressors, mechanical ventilation and other interventions such as dialysis, which places a tremendous strain on medical resources.4 Understanding why some cases result in severe symptoms and how to recognize and treat severity as early as possible could help alleviate some of this burden and reduce the death toll.
What is COVID-19?
COVID-19 is an acronym for COronaVIrus Disease of 2019. It is caused by the novel SARS-CoV-2 virus, which is a member of the Coronaviridae family of viruses, and is closely related to the virus (SARS-CoV) responsible for the severe acute respiratory syndrome (SARS) epidemic that erupted in China in 2003.4 In the majority of cases documented thus far, COVID-19 manifests with similar symptoms to SARS, and is especially notable for its primary complication: severe pneumonia leading to acute respiratory distress syndrome (ARDS).4
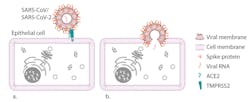
The SARS-CoV-2 genome consists of a single strand of positive-sense RNA5 surrounded by a lipid-bilayer membrane studded with transmembrane proteins. The viral spike protein utilizes the angiotensin-converting enzyme II (ACE2) receptor to enter epithelial cells. Alveolar cells lining the lungs, GI tract, heart and kidney all strongly express the ACE2 receptor and the membrane TMPRSS2 serine protease needed to cleave the viral spike protein and facilitate cell entry. It is not known definitively if organs other than the lungs are directly targeted.5 (Figure 1)
Normal immune response to viral infection
At least 80 percent of those known to be infected with SARS-CoV-2 appear to mount a typical immune response.6 In a normal immune response, a number of different types of immune cells (lymphocytes) and chemical messengers are released in an intricate sequence, causing a mild localized inflammatory state.7 This is triggered when somatic cells are confronted with molecular patterns indicating an invasion or proximity of a pathogen, causing the release of a variety of messenger molecules called cytokines. The first cytokines released are interleukin 1β (IL-1β) and tumor necrosis factor-α (TNF-α), which attract a variety of circulating white blood cells (WBCs) to the infection site, including neutrophils, monocytes, macrophages (monocytes that have migrated into tissues), and natural killer (NK) cells (a type of WBC that can kill infected or neoplastic cells).
This response, along with the antipathogenic chemicals released by these cells (i.e., complement), comprise the innate immune response. These cells directly attack the invading pathogen and also release additional cytokines, chief among them interleukin-6 (IL-6).8 IL-6 is essential for invoking the adaptive immune response, which calls T-cells, B-cells, and T helper (Th) cells to the infection site. IL-6 also stimulates further recruitment, proliferation and activation of macrophages.
Like NK cells, T-cells destroy pathogen-infected cells but are much more specific for the infecting pathogen.9 B-cells produce antibodies that specifically target pathogenic antigens and mark them for elimination. Th cells also release IL-6 and other cytokines, which assist in the process of recruiting and maintaining T- and B-cell activation and proliferation. IL-6 and other cytokines directly cause endothelial cells lining the vasculature and organs to become slightly permeable, which facilitates movement of immune cells and complement into infected tissues.
As infection resolves, anti-inflammatory cytokines help to turn off the proinflammatory response and return the immune system to a nonactivated but vigilant state. Without this regulatory function, the immune system would continue to escalate the proinflammatory response, causing increasing damage to healthy cells and tissues.
Abnormal immunologic response to infection
Overexuberant immunologic responses can lead to irreversible tissue damage. This has been recognized in various clinical areas such as oncology, immunology, infectious disease and rheumatology.8 Nevertheless, it is the ICU physician who is most likely to witness one of the deadliest manifestations of the abnormal immunological response, the cytokine storm syndrome (CSS). This response is also referred to by some as the cytokine release syndrome (CRS).
CSS is characterized by systemic symptoms and signs derived from a massive and uncontrolled inflammatory response caused by pro- and anti-inflammatory cytokine dysregulation.10 It is characterized by continuous activation and expansion of macrophage and lymphocyte populations, which secrete large amounts of cytokines, causing the cytokine storm.11 This massive cytokine release is akin to hemophagocytic lymphohistiocytosis (HLH) disease, a syndrome characterized by initial unchecked and persistent activation of cytotoxic T lymphocytes and NK cells.8
Clinical and laboratory manifestations of HLH include fever, enlarged liver and/or spleen, neurologic dysfunction, coagulopathy, liver dysfunction, cytopenias (i.e., low levels of erythrocytes, leukocytes, and/or platelets), hypertriglyceridemia, hyperferritinemia, hemophagocytosis, and eventually diminished NK cell activity as the immune system becomes progressively paralyzed. HLH can be familial (primary HLH) or secondary to another disease process (sHLH), such as rheumatic disease, in which it is referred to as macrophage activation syndrome (MAS, characterized by elevated ferritin).8
Since blood cell destruction is not mandatory for HLH diagnosis, the boundaries between CSS and sHLH are blurred, and clinical manifestations of CSS can also be triggered by graft-versus-host disease and a specific type of antibody therapy used to treat a variety of cancers.8 CSS also underlies the systemic inflammatory response syndrome (SIRS) that can occur with severe trauma or burns, acute inflammatory diseases such as pancreatitis, and sepsis.9 Viruses, such as herpes and Epstein-Barr are known to trigger CSS, as is H5N1 influenza.7 Current literature indicates that SARS-CoV-2 triggers CSS and has been attributed to the severe symptoms characteristic of critical patients. CSS in these patients is often fatal, as was the case in the previous SARS and MERS outbreaks.10
Considering the diversity of conditions that can trigger CSS, diagnosing the syndrome can be challenging but lifesaving. It should be suspected when there is a worsening clinical condition characterized by unremitting fever, cytopenias, enlarged spleen and/or liver, swollen lymph glands, and/or signs of overwhelming systemic inflammation, without evidence of uncontrolled infection. Laboratory findings of high levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and/or ferritin are suggestive of lymphocyte, phagocytic monocyte and phagocytic macrocyte activation.8
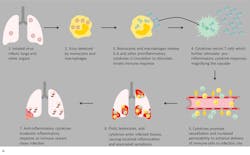
In severe and critical COVID-19 patients, the low number of peripheral lymphocytes in the blood despite a high volume of lymphocytes in the lungs (which are the primary target organs), along with reduced lymphocytes in the spleen, thymus and other lymphoid organs, is indicative of immune exhaustion. This correlates with autopsy findings of widescale immune system destruction, as reflected by atrophy of the spleen and lymph nodes.11 Despite the general lymphocytopenia, surviving T lymphocytes are rapidly activated to become pathogenic Th lymphocytes.
This activation induces inflammatory monocytes to highly express IL-6, starting a localized and then systemic cascade effect that results in hyperproduction of IL-6, which accelerates the inflammatory process.11-13 Because IL-6 also increases vascular permeability, excessive levels cause blood vessels to become very leaky. This, along with clotting factors released from vascular endothelial cells, stimulates the coagulation cascade, resulting in microthromboses (tiny clots), which leads to ischemia and tissue death of the kidney, intestines, heart, liver, brain and extremities.12 (Figure 2a and 2b)
IL-6 as a sensitive CSS biomarker
Multiple biomarker abnormalities are either directly indicative or occur as a consequence of CSS. Consequently, biomarkers can play a central role in the diagnosis, prognosis and monitoring of CSS, as well as detecting and monitoring organ damage arising from CSS.15
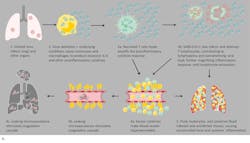
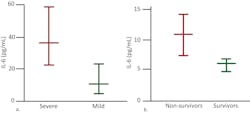
As the causal elements driving the extreme inflammatory process, cytokine assessment can provide valuable information. While proinflammatory cytokines such as IL-1β and TNF-α drive the initial cytokine response, IL-6 dysregulation appears to be the most profound and thus has proved so far to be the best indicator of CSS severity and mortality risk. Chen et al. found that high IL-6 was associated with detectable serum SARS-CoV-2 RNA and poor prognosis.16 Gao et al. demonstrated that COVID-19 patients with severe disease requiring ventilation had statistically significant higher blood levels of IL-6 than patients experiencing only mild symptoms14 and determined that IL-6 was the single best prognostic indicator of severity.15 (Figure 3)
Other markers of inflammation include increased IL-1β, TNF-α, IFN-γ, C-reactive protein (CRP, which elevates due to stimulation by IL-6), erythrocyte sedimentation rate (ESR), and serum amyloid A proteins (SAA). SAA is an early and sensitive marker of the acute-phase inflammatory response and also appears to correlate with disease severity.20 Although procalcitonin (PCT) can be stimulated in response to elevated IL-6, elevated PCT has only been reported thus far in patients with a secondary bacterial infection.15 As tissues sustain injury and other organ systems become affected, other biomarkers that can elevate include ALT, AST, LDH, ferritin, bilirubin, ammonia, myoglobin, creatine kinase and cardiac troponin (cTn).21
Staging and treating COVID-19
Siddiqi et al. proposed a three-stage classification system to define COVID-19 with recommendations on therapy for each stage.4 Stage 1 constitutes infection, incubation, and mild (or absent) nonspecific symptoms suggestive of flu or a cold. These symptoms are typical of the normal immune response invoked by respiratory viral infection. Patients might show some indication of lymphopenia (reduced white blood cells), but do not require medication beyond over-the-counter drugs used to reduce pain and fever. Siddiqi et al. suggest that these patients might benefit from early administration of antiviral drugs—when one is determined to be safe and effective for COVID-19—as these might shorten the intensity and duration of symptoms and possibly prevent progression to more-severe disease.
Several antiviral drugs are currently being studied that have action against other RNA viruses, including already-approved medications such as oseltamivir and baloxavir marboxil/favipiravir used to treat influenza; ritonavir, lopinavir/ritonavir and darunavir, which are HIV protease inhibitors; and cobicistat, which is a cytochrome P450 inhibitor used in combination with darunavir. Investigational drugs are also being explored, such as remdesivir and azvudine, but none have been approved as of this writing.22
The antimalarial drugs chloroquine and hydrodroxychlorquine have also received much interest, as they are reported to possess antiviral activity against a diverse range of other RNA viruses and proved to have therapeutic value in treating SARS. Multiple potential mechanisms have been proposed to explain how these compounds might impede viral replication, viral entry into cells and inflammation in COVID-19. At least 27 clinical trials are proposed or in process worldwide and registered on clinicaltrials.gov; however, none have indicated effectiveness as of yet.
Stage 2 of COVID-19 is classified into two subdivisions.17 Patients in stage 2a have viral pneumonia with cough, fever, and chest imaging that indicates fluid in both lungs (bilateral infiltrates). Patients with stage 2b also have some degree of hypoxia (low blood oxygen). Individuals with either type of stage 2 disease warrant hospitalization, as they can require additional oxygen and should be observed for the development of more-severe symptoms. These patients will usually show signs of systemic inflammation, and laboratory testing can reveal elevated inflammation markers—especially IL-6 that is two or more times greater than the upper limit of normal. Lymphopenia becomes more pronounced as well. Patients who have a secondary bacterial infection might also have elevated PCT. In this stage, Siddiqi et al. suggest the use of anti-inflammatories such as corticosteroids. These must be used with care, however, as they can suppress the immune response and engender increased propagation of the virus.
In stage 3, patients have severe hyperinflammation in the lungs and systemic inflammation associated with sepsis.18,19 This is the stage at which IL-6 is significantly elevated, as are D-dimer and other markers of tissue injury. Stage 3 patients require intensive supportive care measures, and Siddiqi et al. suggest using cytokine inhibitors such as anakinra, which inhibits binding of IL-1 to its receptor, and tocilizumab (ACTEMRA), which prevents IL-6 from binding to its receptor. Both drugs are approved for treating rheumatoid arthritis in adults and children and other select severe inflammatory diseases. (*See author’s note after references)
Currently, 22 studies are registered on clinicaltrials.gov and are either ongoing or enrolling patients. Xu et al. evaluated tocilizumab in 21 COVID-19 patients with severe or critical disease with very positive results. Body temperature was restored to normal within one day of the first dose, and it was possible to reduce oxygen intake for 75 percent of the patients following the first administration, with one patient no longer requiring oxygen therapy at all.23 Michot et al. describe similar results in a case study of a 42-year-old male cancer patient who developed COVID-19. However, the patient was also taking the antiviral combination of lopinavir and ritonavir.
More definitively, Dr. Drew Jones IV, a pulmonologist at Pulmonary Associates of Richmond (Richmond, VA) administered tocilizumab to a 57-year-old male patient diagnosed with COVID-19 who had previously controlled hypertension (personal communication). Two days before administering the drug, the patient’s IL-6 level was more than twice the upper limit of the normal reference range (ULN) determined for the assay by the laboratory. Despite administration of azithromycin, hydroxychloroquine (withdrawn after four doses), and ceftriaxone, the patient deteriorated to stage 2b with expected progression to stage 3 within the following 12-24 hours. IL-6 rose to ~12x ULN.
Following administration of tocilizumab, the patient improved rapidly and significantly and was discharged from the hospital three days later. One confounding factor in this study was that the patient was also administered a very high dose of vitamin C along with the tocilizumab. Dr. Jones believes, however, that timing of therapy is important. He attributes the patient’s rapid recovery to starting the anticytokine therapy before his condition became critical. He has thus far experienced similar responses in over 20 other patients. Dr. Jones is currently engaged in formalizing his study to determine the appropriate time of drug delivery for best effectiveness.
Conclusion
Substantial evidence suggests that COVID-19 severity contributing to severe pneumonia and ARDS, as well as other end-organ damage, is attributable to a severe inflammatory response and cytokine storm syndrome triggered by dysregulated cytokine production—particularly IL-6.20 The central role of IL-6 is reflected in the many controlled clinical trials of cytokine blocking agents being conducted worldwide. Such medications might offer highly effective therapeutic options for treating patients with stages 2b and 3 disease, as might a novel artificial-liver blood-purification system that could remove cytokines from the blood in much the way dialysis is used to filter blood in patients with kidney failure.24 These innovations suggest a promising future in which IL-6 is not only a biomarker that can aid in clinical decision-making but also a target of pharmaceutical and interventional therapies.
References:
- COVID-19 Coronavirus pandemic. 2020 [accessed April 19, 2020]. Available from: https://www.worldometers.info/coronavirus/
- 8 things to know about pandemic influenza. 2020 [accessed April 19, 2020]. Available from: https://www.who.int/news-room/feature-stories/detail/8-things-to-know-about-pandemic-influenza
- Q&A: similarities and differences – COVID-19 and influenza. 2020 [accessed April 19, 2020]. Available from: https://www.who.int/news-room/q-a-detail/q-a-similarities-and-differences-covid-19-and-influenza
- Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. The Journal of Heart and Lung Transplantation. 2020. DOI: 10.1016/j.healun.2020.03.012.
- Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14:e1007236.
- Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T. Host innate immune responses to sepsis. Virulence. 2014;5:36-44.
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16-32.
- Cron RQ, Behrens EM. Cytokine storm syndrome. Springer Nature. Cham, Switzerland. 2019. ISBN 978-3-030-22094-5.
- Systemic inflammatory response syndrome. National Center for Biotechnology Information, U.S. National Library of Medicine. 2020 [accessed April 21, 2020]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547669/
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529-39.
- Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet. 2020. DOI: 10.1016/s0140-6736(20)30920-x.
- Wei H, Xu X, Tian Z, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. National Science Review. 2020. DOI: 10.1093/nsr/nwaa041.
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:M1091.
- Liu T, Zhang J, Yang Y, et al. The potential role of IL-6 in monitoring coronavirus disease 2019. The Lancet. 2020. DOI: 10.2139/ssrn.3548761.
- Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. MedRxiv preprint. 2020 [accessed April 21, 2020].
- Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020. DOI: 10.1002/jmv.25770.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020. DOI: 10.1016/s0140-6736(20)30566-3.
- Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. MedRxiv preprint. 2020. DOI: 10.1101/2020.03.30.20048058.
- Zhang J, Liu Z-H, Luo X-H, et al. Clinical hallmarks of 13 COVID-19 patients revealing SAA biomarker. The Lancet. 2020. DOI: 10.2139/ssrn.3546066.
- Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2020. DOI: 10.1093/eurheartj/ehaa248.
- Coronavirus puts drug repurposing on the fast track. Nature. 2020 [accessed April 21, 2020]. Available from: https://www.nature.com/articles/d41587-020-00003-1
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. chinaXiv:20200300026v1 2020 [accessed April 21, 2020].
- Zhang Y, Yu L, Tang L, et al. A promising anti-cytokine-storm targeted therapy for COVID-19: the artificial-liver blood-purification system. Engineering (Beijing). 2020. DOI: 10.1016/j.eng.2020.03.006.
- Wang X, Xu W, Hu G, et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol 2020. DOI: 10.1038/s41423-020-0424-9.
*Authors’ note: Sirilumab (Kevzara) is another approved IL-6 receptor antagonist currently in phase 2/3 trials for use in COVID-19 patients.
About the Author

H. Roma Levy, MS
holds an MS from UC Santa Cruz in molecular biology with an emphasis in chronobiology, in which she conducted independent research. As a medical writer for Siemens Healthineers, Ms. Levy has written or co-authored multiple articles and clinical educational presentations over the last 16 years in diverse areas, including immunology and infectious disease, endocrinology, cardiology, and opioid addiction.