Glucose meters: current regulatory guidance for manufacturers and providers
Earning CEUs:
For a printable version of the April CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
APRIL LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
- Recall which regulatory agencies are directly involved in the compliance of glucose monitors.
- Discuss the sequence of events and process for the FDA to provide action and insight on the compliance of glucose monitors in healthcare institutions.
- Discuss the process for approval for the use of off-label glucose meters in healthcare institutions.
- Describe the liabilities of the use of off-label glucose meters that healthcare institutions may be subject to.
Therapeutic management of blood glucose in patients with diabetes in the home or in the hospital involves the use of glucose meters for the rapid assessment of whole blood glucose. On Oct. 11, 2016, the U.S. Food and Drug Administration (FDA) published guidance documents for glucose meters.1,2 These guidance documents are for manufacturers, not for providers. The Centers for Medicare and Medicaid Services (CMS) and its designees provide accreditation (certification) for and oversight of provider compliance under the Clinical Laboratory Improvement Amendments (CLIA) of 1988.3 This is an important distinction for all stakeholders involved in the management of dysglycemia in hospitalized patients, many of who are on intensive insulin therapy to achieve safe and effective glycemic control.
The FDA guidance documents make a clear distinction between devices that are designed, cleared, and classified for self-monitoring of blood glucose in the home and devices that are designed, cleared, and classified for blood glucose monitoring for prescription point-of-care (POC) use. It took six years for the FDA to publish these guidance documents for manufacturers. The initial process involved an open forum on March 16 and 17, 2010, followed by publication of draft guidance documents in January of 2014, which were circulated for public comment and finalized in October of 2016.1,2 A subsequent FDA advisory panel meeting held on March 30, 2018, addressed the use of capillary whole blood testing with a glucose meter in vulnerable patient populations in acute care facilities.4 The trigger for the FDA’s actions was the compilation of adverse events including numerous deaths in its MAUDE (Manufacturer and User Facility Device Experience) database.5 These events occurred when self-monitoring blood glucose test systems (SMBG) for over-the-counter (OTC) use migrated into the hospital. During the six years when the FDA was developing new guidance for manufacturers, there was great debate about the performance criteria for glucose meters. Unfortunately, criteria were established by the International Organization for Standardization (ISO), Clinical and Laboratory Standards Institute (CLSI), FDA, and other standards organizations without sufficient evidence about device performance in all patient care settings with all specimen types by CLIA-waived operators.
The FDA’s publication of two separate guidance documents clearly separates devices into two classes:
- “Self-Monitoring Blood Glucose Test Systems (SMBG) for Over the Counter (OTC) Use” and
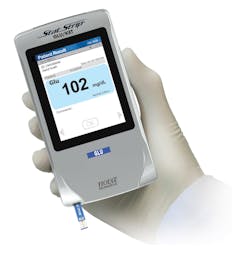
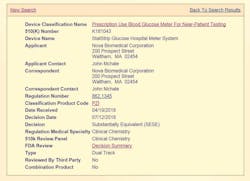
- “Blood Glucose Monitoring Test Systems (BGMS) for Prescription Point-of-Care (POC) Use” (Figures 1, 2, and 3). To date, there is only one device that is cleared and classified by the FDA as a Blood Glucose Monitoring System (BGMS) for Prescription Point-of-Care (POC) Use (Figure 4).6,7
Subsequently, and after the FDA’s Advisory Panel4 on capillary whole blood, the same device was cleared (k181043) for use by CLIA-waived operators with all patients in all healthcare settings with all specimen types. The device was then classified by the FDA as the only “Prescription Use Blood Glucose Meter For Near Patient Testing” (Product Code PZI).6,7
The FDA had established a clear pathway for clearance of devices based on the evidence submitted by this manufacturer over several significant submissions drawing from very large prospective and retrospective (Real World Evidence) datasets, with a combined N=>20,000 paired glucose measurements comparing arterial, capillary, and venous whole blood to central laboratory traceable plasma glucose results. These clearances and the new classification have profound implications for use by providers under CLIA ‘88.
Use of other devices classified as OTC SMBG (Product Code NBW) based on the FDA guidance is considered off label in hospitals14 because these devices have not been evaluated for use in these facilities and, specifically, in vulnerable populations such as critically ill patients.
Off-label use under CLIA ‘88 requires providers to restrict the use of these devices to CLIA-waived operators. This has significant operational considerations within hospitals as to who can perform testing in vulnerable patients including critically ill. When a provider chooses to use a glucose meter off label, the hospital and laboratories must possess a CLIA Certificate of Compliance (CoC) or Certificate of Accreditation (CoA), establish the performance specifications (i.e. accuracy, precision, analytical sensitivity, specificity including interfering substances) and other performance characteristics for use in their patient populations, and meet the additional CLIA regulatory requirements for high-complexity testing as well as any applicable state regulations. Compliance with these rules, however, does not eliminate the off-label use of a glucose meter. In addition to patient safety concerns, the off-label use of a glucose meter in critically ill patients raises regulatory and legal concerns that may have important implications for both providers and patients.
Consequences under CLIA
Because the conditions for CLIA-waived status (that a test be simple and have a low risk of erroneous results) have only been demonstrated for the test when used according to its labeling:
- Glucose meters lose their status as CLIA-waived tests when used off label and are considered high complexity tests.
- If a facility wishes to use a BGMS off label (e.g., in a critically ill patient population when a manufacturers’ instructions contain a limitation on critically ill patients), laboratories with a [CLIA waiver] may:
a. Obtain a CoC or CoA
b. Establish performance specifications [(i.e. accuracy, precision, analytical sensitivity, analytical specificity including interfering substances, reportable range of test results, reference intervals, and any other performance characteristic required for test performance) for use in their patient population]; and
c. Meet the additional CLIA regulatory requirements for high-complexity testing and any applicable State regulations. - A laboratory/hospital performing high complexity testing that doesn’t meet the requirements is subject to a notice of deficiency.
a. This requires submitting a corrective action plan to CMS or its designee, the College of American Pathologists (CAP).
b. CMS can suspend or revoke a Certificate of Waiver (CoW).
c. CMS can cancel a provider’s approval for Medicare reimbursement for failure to comply with the corrective action plan in a timely manner.
Because only a BGMS has been cleared and classified by the FDA for use in vulnerable patient populations, the off-label use of other glucose meters with critically ill patients could also present legal risk for providers.
Legal consequences include but are not limited to:
Liability for patient negligence
- Tort liability for negligence could occur if facility physicians, nurses, staff, or laboratories use a glucose meter off label with a critically ill patient and an inaccurate reading results in patient injury.
- Off-label use of a glucose meter could be used as evidence that providers breached the standard of care,particularly in light of safety communications on this topic issued by the FDA, CMS, and other agencies.
- The doctrine of informed consent requires that providers disclose the nature of a proposed procedure and its benefits and risks, as well as any feasible alternatives.
a. If a provider uses a glucose meter off label and does not inform a patient of the known risks of an inaccurate glucose reading, there is a risk that the patient’s consent does not meet the informed consent standard.
b. Under the theory of respondeat superior or vicarious liability, the medical facility could be liable for these and other actions of its physicians, nurses, staff, or laboratories with respect to the off- label use of glucose meter in critically ill patients.
Liability for hospital corporate negligence
- In some states, medical facilities could also be directly liable for a patient’s injuries, independent of the actions of its providers.
- Under the corporate liability doctrine, hospitals have a duty to oversee the practice of medicine within its walls.
- The fact that the majority of U.S. hospitals choose FDA-cleared devices for testing in their critically ill patients is one that courts could consider when assessing whether a hospital using a glucose meter off label has satisfied a reasonable standard of care for its patient.
Liability under CLIA ‘88 and compliance under:
- CLIA waiver:
a. Conditions for CLIA-waived status
b. Glucose meter loses its status as CLIA-waived test if used off label - CMS’s guidance (risk to Medicare reimbursement [loss])
a. High complexity testing not properly performed under CLIA ‘88 - State guidance, regulations, non-compliance actions impacting accreditation and possibly reimbursement. Agencies include but are not limited to:
a. New York and Washington State health departments
b. The Joint Commission
c. CAP
d. ECRI
Conclusion
In summary, the off-label use of a glucose meter or any medical device can result in charges against providers and hospitals for negligence and medical malpractice as a result of not following the doctrine of informed consent or maintaining the standard of care, which can result in medical board investigations. Non-compliance with CMS can possibly affect accreditation (license) and reimbursement including liability for false claims.
The StatStrip Glucose Hospital Meter System is a professional BGMS for prescription POC use that is cleared and classified (Produce Code PZI) for use in all patient care settings with all specimen types by CLIA-waived staff.
As of today, based on device limitations and NBW classification, the use of all other glucose meters in acute care facilities is considered off label by the FDA. The patient safety, legal, and regulatory consequences for off-label use are significant and should be avoided. It is important for institutions to consult their risk management and legal offices regarding off-label use of a glucose meter.
For a printable version of the April CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
REFERENCES
- U.S. Food and Drug Administration, Center for Devices and Radiological Health. Blood glucose monitoring test systems for prescription point-of-care use; Guidance for industry and Food and Drug Administration staff. Silver Spring, MD: 2016. http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380325.pdf
- U.S. Food and Drug Administration, Center for Devices and Radiological Health. Self-monitoring blood glucose test systems for over-the-counter use; Guidance for industry and Food and Drug Administration staff. Silver Spring, MD: 2016. https://www.fda.gov/downloads/ucm380327.pdf
- U.S. Food and Drug Administration, Center for Devices and Radiological Health. Recommendations for Clinical Laboratory Improvement Amendments of 1988 (CLIA) waiver applications for manufacturers of in vitro diagnostic devices; Guidance for industry and Food and Drug Administration staff. Silver Spring, MD: 2008. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm070890.pdf
- U.S. Food and Drug Administration. FDA executive summary, meeting of the Clinical Chemistry and Clinical Toxicology Devices Panel. 30 Mar 2018. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/ClinicalChemistryandClinicalToxicologyDevicesPanel/UCM602692.pdf
- U.S. Food and Drug Administration. Manufacturer and User Facility Device Experience Database—(MAUDE). https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/PostmarketRequirements/ReportingAdverseEvents/ucm127891.htm
- U.S. Food and Drug Administration. 510(k) Substantial equivalence determination decision summary, k132121: 2016. https://www.accessdata.fda.gov/cdrh_docs/reviews/K132121.pdf
- U.S. Food and Drug Administration. 510(k) Substantial equivalence determination decision summary, k181043: 2018. https://www.accessdata.fda.gov/cdrh_docs/reviews/K181043.pdf
- DuBois JA, Slingerland RJ, Fokkert M, et al. Bedside glucose monitoring–Is it safe? A new, regulatory-compliant risk assessment evaluation protocol in critically ill patient care settings. Crit Care Med 2017;45(4):567-574. doi: 10.1097/CCM.0000000000002252.
- Rice MJ, Smith JL, Coursin DB. Glucose measurement in the ICU: Regulatory intersects reality [Editorial]. Crit Care Med 2017;45(4):741-743. doi: 10.1097/CCM.0000000000002274
- DuBois JA, Slingerland RJ, Fokkert M, et al. Does regulatory really intersect reality in glucose measurement in the ICU? Is the issue testing method accuracy or specimen type? Crit Care Med 2017;45(11):e1186-e1188. doi: 10.1097/ccm.0000000000002596
- Klonoff DC, Draznin B, Drincic A, et al. PRIDE statement on the need for a moratorium on the CMS plan to cite hospitals for performing point-of-care capillary blood glucose monitoring on critically ill patients. J Clin Endocrinol Metab 2015;100(10):3607-3612. doi: 10.1210/jc.2015-2435
- Klonoff DC, Vigersky RA, Nichols JH, et al. Timely hospital glucose measurement: Here today, gone tomorrow? Mayo Clinic Proc 2014;89(10):1331-1335. doi: 10.1016/j.mayocp.2014.08.005
- Rice MJ, Coursin DB. Glucose meters: Here today, gone tomorrow? Crit Care Med 44(2):e97-e100. doi: 10.1097/CCM.0000000000001389
- Landree L. Final guidance documents: Self-monitoring blood glucose test systems for over-the-counter use and blood glucose monitoring systems for prescription point-of-care use. Center for Devices and Radiological Health webinar: 21 Nov 2016. https://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm525364.htm
About the Author