Recent years have produced a wealth of discovery in the molecular diagnostics (MDx) field, as the identification of new genes and mutations has pointed to clinically relevant diagnostic, prognostic, and therapeutic information. The application of newly developed targeted therapies is increasingly driven by molecular mutational analysis that indicates the patient’s response or resistance to those therapies. In this article, I’ll discuss one particular application of molecular tests that have become key diagnostic indicators in two substantial hematopoietic disease classifications, i.e., myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN); the economics facing these diagnostic indicators; and the clinical impact observed.
MDS and MPN account for approximately 40 percent of all hematopoietic cancers. The National Comprehensive Cancer Network guidelines recommend testing for the JAK2, MPL, and CALR genes1-3 as part of the diagnostic workup of patients being assessed for MDS or MPN, to provide both diagnostic and prognostic guidance in treating the patient. Labs are now expected to incorporate molecular workups into a routine evaluation of a bone marrow biopsy, as well as with peripheral blood specimens, to provide a comprehensive evaluation of the specimen, and provide the treating clinician with a complete picture of the patient’s diagnosis and prognosis. These molecular tests play a critical part in that comprehensive evaluation.
Molecular test economics
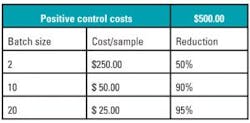
Like any test, the evaluation of molecular markers requires both testing reagents as well as negative and positive controls. The cost of these reagents (and in particular, the controls) is often influenced by how often the mutation presents itself clinically. The less frequent the mutation is, the more challenging it is to create positive controls, hence the more expensive they become. Since a positive control is required per run and not per each sample testing, in order to reduce the overall costs of the tests, laboratories will typically batch samples. Given the low frequency of mutations in the JAK2, MPL, and CALR genes, the corresponding reagents and controls are increasingly expensive. Table 1 provides a simple mathematical example to illustrate this. While batching is a sensible economic decision in order to increase the profitability of a lab, there is a negative potential clinical impact on the turnaround time provided to a clinician who awaits their patient’s diagnosis.
Workup timeline
A typical bone marrow workup requires between seven to 10 days, and will include a morphologic assessment, histology, flow cytometry, and cytogenetics testing. As the diagnosis is being identified, molecular tests are often added to complete the diagnostic picture. Upon surveying the leading labs, we found that the molecular workup for these three genes takes between two to three weeks. This means that, in order for a pathologist to include the results of the molecular tests in the final workup, the results are often delayed by two to three weeks. For a clinician awaiting the diagnosis to begin treatment, this is a significant delay; for the patient facing the diagnosis of a deadly disease—an eternity.
Solutions
While these mutations (JAK2, JAK2 exon 12, MPL, and CALR) are important to obtaining a full diagnostic picture, the data shows that they present in patients quite infrequently; more than 80 percent of patients return a negative answer on these molecular tests.4-6 A screening test would be beneficial in identifying those negative patients; and if this screen was low-cost, thus lowering the batching threshold, laboratories could, while maintaining reasonable economics, provide a quick response to the majority of patients which have a negative result. Furthermore, if the screen were to indicate which genes were positive, a follow-up confirmation would be substantially less expensive since it would require both infrequent testing (one in five patients, or the remaining 20 percent who are positive); and it would only require testing one of the three genes. This would result in a 1:15 reduction in the overall cost factor for the positive confirmation.
A potentially significant advance in this area has been made with the recent commercial launch of a novel, proprietary test, HemeScreen, which screens for mutations in the JAK2, JAK2 exon 12, MPL, and CALR genes. The test is offered to hospital labs as a send-out test in our CLIA lab. This assay is capable of delivering negative results within two days, and positive results within five days. Precipio offers both commercial insurance and government payor billing for the test, as well as direct bill, providing the hospital with a financial benefit.
Technological innovations suggest an exciting future as the molecular diagnostics field seeks solutions that are both more efficient, providing a faster answer to the clinician and their patient, and are substantially more cost-effective than those currently available by other providers.
REFERENCES
- National Comprehensive Cancer Network. NCCN publishes new clinical practice guidelines for myeloproliferative neoplasms. Available at: https://www.nccn.org/patients/foundation/newsdetail.aspx?NewsId=801
- National Comprehensive Cancer Network. NCCN guidelines for patients: myeloproliferative neoplasms. 2018:20-21. Available at: https://www.nccn.org/patients/guidelines/mpn/20/
- Mesa R, Jamieson C, Bhatia R, et al. Myeloproliferative neoplasms, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016 Dec;14(12):1572-1611.
- Jekari DW, Han SB, Kim M, et al. JAK2 V617F mutation in myelodysplastic syndrome, myelodysplastic syndrome/myeloproliferative neoplasm, unclassifiable, refractory anemia with ring sideroblasts with thrombocysts, and acute myeloid leukemia. Korean J Hematol. 2010 Mar;45(1):46-50.
- Steensma DP, Dewald GW, Lasho TL. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005 Aug 15;106(4):1207-1209.
- Fermo E, Zaninoni A, Imperiali FG, et al. Analysis of JAK2 V167F mutation in myelodysplastic syndromes. Blood. 2007;110:4591.
About the Author

Ilan Danieli
serves as CEO of Precipio, Inc., and is the developer of HemeScreen, a novel proprietary test for mutations in hematologic cancers.