A novel point-of-care approach for improving acute bleeding management
Whole blood viscoelastic testing (VET) for perioperative bleeding management is systematically increasing in clinical use and is approaching the level of standard of care for many clinical settings such as cardiovascular surgery, liver transplantation, trauma, and obstetric hemorrhage. Conventional coagulation testing has proven to be inadequate for directing therapeutic intervention in these critical settings.1-5
Physicians managing acute bleeding events require faster turnaround times for test results and prefer assays that more accurately reflect the whole blood or cell-based hemostatic process described by Hoffman and Monroe.6 The benefits of VET have been well-documented. There exists an abundance of publications and systematic reviews in a variety of clinical settings,7-10 including review articles in this area published in previous issues of MLO.11-13 Several medical societies have given strong recommendations for the use of VET in conjunction with goal-directed treatment algorithms guided by VET for managing acute bleeding in the perioperative setting.14-17 To date, two technologies have emerged at the forefront of whole blood VET: thromboelastography and rotational thromboelastometry. Both employ an adaptation of the classic methodology, first reported by Hartert in 1948,18 involving a pin suspended in a cup containing a whole blood sample. Despite some advancements in the testing process and various modifications of the assays, the clot detection methodology using a cup and pin shared by these two systems has remained relatively unchanged for over seventy years.
The objective of this article is to review the classic VET clot-detection principles and to describe a novel approach with a new point-of-care (POC) hemostasis platform that may offer a higher resolution of clot detection, a simplified testing process and an intuitive results output that rapidly provides actionable information, thus potentially making clinical application and interpretation easier.
Classic VET method
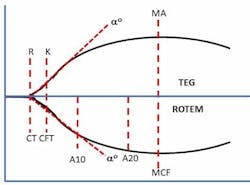
Classic VET methods detect the onset of clot initiation and the increasing development of clot stiffness by measuring the resistance to the movement between two surfaces. These surfaces are provided by a small cup containing whole blood and reagents and a pin that is inserted into the blood-filled cup. The pin displaces the blood and reagent sample to create a one mm gap between all surfaces. Depending on the technology, either the cup or pin rotates with a slight degree (4.45° to 4.75°) at a rate of five to six times per minute. As the activated blood begins to clot, it forms connections between the surfaces of the cup and pin. These connections are fractured by the mechanical movement of the surfaces until the clot develops enough strength to resist fracture and increasingly impede the movement of the cup and pin surfaces. The measured increase in clot stiffness is then graphically displayed as a curve (Figure 1) from which numerous parameters are derived that represent various aspects of the coagulation process. These parameters assess clot initiation, which reflects the length of time (in seconds or minutes) it takes for a nascent clot (representing initial fibrin formation) to be detected. The rate of fibrin polymerization or clot amplification is determined by the length of time (in seconds) the clot takes to increase from 2 mm to 20 mm of clot stiffness.
Next, overall clot stiffness, which reflects the contributions of fibrinogen and platelets, is measured at various times until a maximum level (measured in mm) is achieved. Interestingly, the use of millimeters as a measure of the viscoelastic properties of a clot has no physical meaning in this context. It was simply the original method used for recording the range of pin movement on a film that was 100 mm wide. Hartert arbitrarily assigned values of 0 to 100 mm for the measurement range.19 Finally, various fibrinolysis parameters are subsequently measured as clot stiffness (in percent lost or percent remaining) diminishes due to normal or pathological process. Several comprehensive reviews of both currently available technologies, including system descriptions, features and limitations, have previously been published.20-23
Using the classic methods, VET is a relatively laborious process that is fraught with potential causes of preanalytical errors. These methods require multiple pipetting steps and the open transfer of blood from the collection tube to the measuring cups, factors that limit their acceptance at the POC. Additionally, the measurement of the sample may be disrupted due to susceptibility to environmental disturbances such as vibration or movement and drying artifact because of exposure to air. Finally, the ability to interpret the graphical output and the numerous parameters that are reported requires individual dedication to education and interpretation training. The steep learning curve, combined with the operational complexities of more classical VET, provides an impediment to broader use by physicians, and this may limit the clinical benefits to narrower patient populations.
Novel ultrasound technology
In recent years, different technologies utilizing novel clot-detection methods have emerged. These technologies employ a variety of different principles to generate vibration and are detected and related to changes in viscoelastic properties as a function of the coagulation and fibrinolysis properties of the sample. One of the novel devices uses a patented, “ultrasound” technology called SEER (Sonic Estimation of Elasticity via Resonance) sonorheometry.24 The use of ultrasound is common in medical practice, with ultrasound-based imaging devices being utilized across a broad spectrum of patient populations. Ultrasound signal processing techniques are also employed to characterize changes in mechanical properties of soft tissues that might be indicative of diseased tissues. This growing area of clinical research is called elastography.25
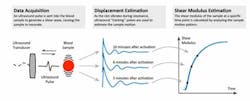
SEER sonorheometry uses high-frequency ultrasound pulses like those used in diagnostic imaging to measure changes over time in shear modulus, a direct physical measure of clot stiffness (Figure 2). The utilization of ultrasound allows the sample to be interrogated without having physical contact with moving parts, thereby reducing the potential interference with the coagulation process as occurs in classic VET methods. This results in increased sensitivity to early clot formation and increased sensitivity to soft clots, which are often those associated with clinical bleeding. Furthermore, in contrast to other VETs, the direct measurement of the sample shear modulus allows accurate estimation of the relative contributions of platelets and fibrinogen to overall clot stiffness.26
Expanded testing at the POC
As new technology for bleeding management is introduced to the clinical market, the primary goal is to not compromise clinical performance in exchange for testing automation, speed, and space-saving design. However, while analytical performance is paramount, important considerations must be given to reducing the complexity of the testing process and simplifying the interpretation of the results. Improving these aspects of VET is key to expanding this type of testing to a broader range of clinical settings and move it closer to the POC.
Relative to the primary objective of clinical performance, one analyzer has been evaluated and compared to classic VET methods in a variety of clinical settings including cardiac surgery,27-28 spine surgery,29 and other critical care settings30 and has demonstrated good correlation with similar clotting parameters reported by standard laboratory testing and good concordance with the clinical presentation (i.e., bleeding or non-bleeding). Second, cartridge-based systems have automated the testing process by eliminating the need to openly transfer blood from the collection tube or to manually mix reagents. This reduces the chance for untimely pre-analytical processing errors that can delay the time to obtain results needed to treat patients. Furthermore, this offers the opportunity to use the system by nonlaboratory healthcare personnel at the point of care. Improvements in automating steps in VET have been proven to reduce inter-operator and intra-operator variability of results in multiuser settings.31 In addition, automation of VET can presumably help to reduce testing costs by reducing product waste from errant testing.
Finally, with a unique and intuitive display (Figure 3), the interpretation of test results can be made easier for less experienced clinicians to arrive at the correct conclusions about enzymatic factor deficiencies (CT = clotting time), platelet or fibrinogen dysfunction (CS = clot stiffness, PCS = platelet contribution of CS, FCS = fibrinogen contribution to CS), or the influence of heparin (CTH = CT with heparinase, CTR = CT:CTH ratio) that may cause coagulopathic bleeding. This new display incorporates dials that are ubiquitous in everyday life. Clinicians can easily detect coagulation characteristics that may clarify the reason for bleeding or thrombosis or portend an increased risk for either condition.
Summary
VET devices have been proven to be effective tools to help manage perioperative bleeding and improve patient outcomes by enhancing the physician’s ability to stop bleeding quickly and thereby reduce unnecessary transfusions and complications associated with transfusions. Numerous physicians and researchers using the classic VET technologies of thromboelastography and thromboelastometry have done much of the heavy lifting to challenge convention and to transform acute bleeding management in many clinical settings. The challenges now for companies with novel approaches are to improve on the clot-detection methods, to create more user-friendly technologies, and to provide uncompromised clinical results that can be easily performed by clinicians in clinical settings moving ever closer to the patient. The benefits of the emergence of these new technologies is better hemostasis management with faster diagnostics and easier clinical application. This can now be realized for more patients who experience bleeding complications than ever before.
REFERENCES
- Haas T, Spielmann N, Mauch J, et al. Reproducibility of thrombelastometry: Point-of-care versus hospital laboratory performance. Scand J Clin Lab Invest. 2012;72:313-317.
- Akay OM. The double hazard of bleeding and thrombosis in hemostasis from a clinical point of view: a global assessment by rotational thromboelastometry (ROTEM). Clin. Appl. Thromb. Hemost., 2018 Jan 1.
- Smart L, Mumtaz K, Scharpf D, et al. Rotational thromboelastometry or conventional coagulation tests in liver transplantation: comparing blood loss, transfusions, and cost. Ann Hepatol. 2017;16:916-923.
- Saner FH, Abeysundara L, Hartmann M, Mallett SV. Rational approach to transfusion in liver transplantation. Minerva Anestesiol. 2018; 84:378-388.
- Snegovskikh D, Souza D, Walton Z, et al. POC viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J Clin Anesth. 2018;44:50-56.
- Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb. Haemost. 2001;85:958-965.
- Franchini M, Mengoli C, Cruciani M, et al. The use of viscoelastic haemostatic assays in non-cardiac surgical settings: a systematic review and meta-analysis. Blood Transfus.2018;16:235-243.
- Deppe AC, Weber C, Zimmermann J, et al. POC thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. J. Surg. Res. 2016;203:424-433.
- Veigas PV, Callum J, Rizoli S, et al. A systematic review on the rotational thrombelastometry (ROTEM) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med.2016;24:114.
- Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography or thromboelastometry to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016;8:CD007871.
- Allen TW. Thromboelastometry: Its method, application and benefits. MLO. 2014;46:26-29.
- Allen TW. Clinical application of thromboelastometry in cardiovascular and thoracic surgery. MLO. 2015;47:20-22.
- Allen TW, Sheldahl K. Meeting thromboelastometry clinical needs and new quality standards. MLO. 2016;48:20-22.
- Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53:79-111.
- Shaylor R, Weiniger CF, Austin N, et al. National and international guidelines for patient blood management in obstetrics: a qualitative review. Anesth. Analg. 2017;124:216-232.
- Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology. 2015;122: 241-275.
- Kozek-Langenecker SA, Ahmed AB, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol. 2017;34:332-395.
- Hartert H. Blutgerinnungsstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. Klin Wochenschr. 1948;26:577-583.
- Hochleitner G, Sutor K, Levett C, et al. Revisiting Hartert’s 1962 Calculation of the Physical Constants of Thromboelastography. Clinical and Applied Thrombosis/Hemostasis 2017;23(3):201-210.
- Bolliger D, Tanaka KA. POC coagulation testing in cardiac surgery. Semin Thromb Hemost. 2017;43:386-396.
- Williams B, McNeil J, Crabbe A, Tanaka KA. Practical use of thromboelastometry in the management of perioperative coagulopathy and bleeding. Transfus Med Rev. 2017;31:11-25.
- Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012;26:1-13.
- Othman M, Kaur H. Thromboelastography (TEG). Methods Mol Biol. 2017;1646:533-543.
Corey FS, Walker WF. Sonic estimation of elasticity via resonance: a new method of assessing hemostasis. Ann Biomed Eng. 2015;44:1405-1424. - Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94:487-495.
- Solomon C, Ranucci M, Hochleitner G, et al. Assessing the methodology for calculating platelet contribution to clot strength (platelet component) in thromboelastometry and thrombelastography. Anesth Analg. 2015;121:868-878.
- Huffmyer JL, Fernandez LG, Haghigian C, et al. Comparison of SEER sonorheometry with rotational thromboelastometry and laboratory parameters in cardiac surgery. Anesth Analg. 2016; 123:1390-1399.
- Reynolds PS, Middleton P, McCarthy H, Speiss B. A comparison of a new ultrasound-based whole blood viscoelastic test (SEER sonorheometry) versus thromboelastography (TEG) in cardiac surgery. Anesth Analg. 2016;138:96-102.
- Naik BI, Durieux ME, Knisely A, et al. SEER sonorheometry versus rotational thromboelastometry in large volume blood loss spine surgery. Anesth Analg. 2016;123:1400-1407.
- Ferrante EA, Blasier KR, Givens TB, et al. A novel device for the evaluation of hemostatic function in critical care settings. Anesth Analg. 2016;123:1372-1379.
- Anderson L, Quasim I, Steven M, et al. Interoperator and intraoperator variability of whole blood coagulation assays: a comparison of thromboelastography and rotational thromboelastometry. J Cardiothorac Vasc Anesth. 2014;28:1550-1557.
Special thanks to colleagues Debbie Winegar, PhD. Wim Houdijk, PhD, and Jeffery Light for valuable review and edits.