New ways of approaching sepsis will improve patient outcomes
Earning CEUs:
Sepsis stems from the dysregulation of a host’s inflammatory response to an infection. It is estimated that approximately 1.5 million cases are documented within the United States annually.1 The associated physiologic and biochemical changes often lead to multiple organ dysfunction, with death as a frequent outcome. The syndrome carries a high mortality rate, claiming the lives of approximately 258,000 people per year. A look at the rates of 27 U.S. hospitals from 2005 to 2014 showed an incremental case increase from 15 to 18.6 per 1,000 hospital admissions, with a corresponding mortality rate of 51 percent.2
From an economic standpoint, sepsis places a severe burden on the U.S. healthcare system. It is the most expensive inpatient condition to treat, with annual costs of approximately $24 billion (in 2013). That year, that amount accounted for 6.2 percent of all hospital-related costs; the average patient hospitalization cost over $18,000. The overall observed increase in cases has been attributed to individuals living into older age, with approximately 60 percent to 85 percent of sepsis cases occurring in patients older than 65 years.3 Globally, a concerted effort has been made to raise sepsis awareness, as projections indicate that the incidence and associated costs of sepsis will continue to increase in the future.
The changing definition of sepsis
The definition of sepsis has evolved over time. Beginning with Sepsis-1 at a 1991 consensus conference, it was developed around the Systemic Inflammatory Response Syndrome (SIRS) criteria. Sepsis-1 was defined as infection or suspected infection leading to SIRS. These criteria included a heart rate greater than 90 beats per minute, a breathing rate greater than 20 breaths per minute, a body temperature greater than 38 degrees or less than 36 degrees Celsius, and a white blood cell count greater than 1200/mm or bandemia greater than 10 percent. Sepsis-2 expanded on Sepsis-1 criteria, but maintained the need for at least two of the SIRS criteria in addition to a suspected or confirmed infection. The most recent Sepsis-3 definition, as of 2016, consists simply of life‐threatening organ dysfunction caused by a dysregulated host response to infection.4
Role of the host immune response
The host immune response plays a crucial role in the progression of sepsis and thus has been an area of increasing study. A patient’s immune system is the first line of defense against offending pathogens and is composed of an innate as well as an adaptive system. The innate system is triggered by foreign pathogens but does not exhibit any memory to antigens, whereas the adaptive system does exhibit memory to prior pathogen stimuli.
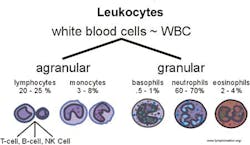
The innate system functions through pattern recognition receptors located on the surface of cells that in turn have the capability to initiate signaling cascades responsible for the generation and release of inflammatory cytokines. It is composed of natural killer cells, mast cells, eosinophils, basophils, macrophages, neutrophils, and dendritic cells. These coordinate within the immune system to identify and eliminate potentially infectious pathogens (Figure 1).
In comparison, there are two types of adaptive responses: humoral immunity, via antibody production by B-lymphocytes, and cell-mediated immunity, by T-lymphocytes. Activated monocytes have been associated with adaptive immunity and antigen presentation. In addition to their role in the immune response against infection, they have been implicated in the pathogenesis of several inflammatory disorders, including sepsis.5
Limits of microbial detection
As the diagnosis of sepsis is often difficult due to patients having varied presentations, diagnosis generally calls for a combination of clinical, laboratory, and imaging-based modalities. Clinical history provides insight, as there are a number of comorbidities that increase a patient’s risk for sepsis. These include obesity, chronic diseases such as diabetes and HIV, and other disease states such as cancer. Of all the comorbidities, the most significant risk factor appears to be hematologic cancers.6
Microbial culture has long been the standard for the detection of bacteria in support of making a clear diagnosis. However, traditional culture tends to be a time-consuming process that is marred by a high rate of false negatives, particularly in the case of slow‐growing organisms or in patients with ongoing antimicrobial regimens. Estimates as low as 30 percent have been quoted for conventional techniques’ ability to detect offending microbes in patients with known infectious sources. Upwards of 25 percent of patients are found to be culture‐negative. In cases where detection does occur, it has been estimated that gram‐positive organisms account for 46.8 percent of infections, with gram negatives at 62.2 percent, fungi at 19.4 percent, anaerobes at 4.5 percent, and parasites at 0.7 percent. Patients may carry more than one infectious organism. The most common inciting sites of infection are respiratory, bacteremia (site unspecified), genitourinary, abdominal, and wound/soft tissue, in that order.7
In recognition of the limits of microbial detection, there is growing interest in potential alternatives. Among alternatives being investigated are technologies including PCR, microarray analysis, bacteriophage, and microfluidics, including flow cytometry and immunoassays. Another area of interest has been implantable devices such as central venous catheters that are embedded with diagnostic capability. These “smart venous catheters” use the electrical impedance characteristics of bacterial biofilm formation to detect microbial growth.8
Clinical use of biomarkers
To date, approximately 200 biomarkers have been studied in the evaluation of sepsis. The most frequently used biomarkers are C-reactive protein (CRP), procalcitonin (PCT), and lactate. CRP is synthesized by the liver in response to signaling factors released by macrophages. It can increase 1000-fold in the blood in response to inflammation and infection. The normal test result should be less than 10 milligrams per liter (mg/L).9 PCT is a precursor to calcitonin, a hormone associated with calcium levels. Its concentration is elevated in circulation in response to inflammation, and in this situation it is not cleaved to calcitonin. Although higher PCT concentrations tend to suggest a systemic bacterial or fungal infection, these levels do not correlate with the severity of sepsis or with mortality.10 PCT has been increasingly used to trend response to therapy within clinical settings.
Serum lactate is another tool that is being used as a more sensitive marker for potentially impending septic shock. Lactate is formed from the reduction of pyruvate, which is generated largely by anaerobic glycolysis. In tissue hypoxia, lactate is overproduced via increased anaerobic glycolysis and worsens if liver dysfunction and acute kidney injury occur due to a decreased ability to clear lactate from the body.11
Viral and fungal detection
The majority of sepsis cases are bacterial, and the discernment of bacterial from fungal, or in rare cases viral, sepsis is of importance for appropriate therapy selection and antimicrobial stewardship. Although rare, viruses, particularly influenza viruses, are increasingly a cause of severe sepsis, either directly or as a result of post-viral secondary bacterial infections.12
To this end, biomarkers such as IFI27, MxA, 1-3-β-D‐glucan, and ASPAG are being investigated for their clinical utility. For the IFI27 biomarker, an increased IFI27 gene expression in peripheral blood indicates an immune response to particular respiratory viruses.13 As for the myxoma resistance protein 1 (MxA), it is induced during viral infections as well, so MxA testing could be helpful in differentiating between viral and bacterial infections. MxA has also been investigated as a potential viral infection marker in children, at least with RSV and rotavirus.14
Invasive aspergillosis (IA) is a fungal infection that particularly affects immunocompromised hosts. Several studies have indicated a high incidence of IA in ICU patients. Additionally, prospective surveillance studies among transplant recipients have shown IA to be the most common type of fungal infection among stem cell transplant recipients and the second‐most common type of fungal infection among solid organ transplant recipients. The U.S. Food and Drug Administration (FDA) approved the detection of galactomannan, a molecule found in the cell wall of Aspergillus species. Serum monitoring of galactomannan can potentially allow initiation of antifungal therapy before life‐threatening infection occurs.15 1-3-β-D‐glucan (BDG) offers another potential target, because it is found as a cell wall component in most fungi, with the exception of the cryptococci, the zygomycetes, and Blastomyces dermatitidis.16
Cytokines and cell surface receptors
Inflammatory marker investigations have been difficult because these markers are shared across a variety of inflammatory conditions and disease states. Cytokine levels for TNF Alpha, IL-6, IL-8, and IL-1B have been shown to elevate as part of the septic response. The most studied of these is IL-6, a pro‐inflammatory cytokine produced by lymphocytes, fibroblasts, and monocytes. It has been suggested that IL-6 concentration in sepsis is the best marker of the severity and outcome for sepsis, because it serves as an important mediator during the acute phase response to inflammation in sepsis.17 In regard to cell surface receptors, one leading candidate is neutrophil CD64 expression, which has so far shown good performance as a potential diagnostic marker in the evaluation of infection and sepsis.18
Other promising biomarkers
Inflammatory response cells like monocytes and neutrophils are also showing promise as aids to diagnosis. Monocytes are large leukocytes that circulate in the peripheral blood and are recruited into tissues at sites of inflammation, where they differentiate into large phagocytic cells called macrophages. Crouser et al’s feasibility study showed that monocyte distribution width (MDW), compared with other potential early sepsis parameters, best distinguished sepsis from SIRS and severe sepsis from noninfected ED patients, with a sensitivity of 77 percent, specificity of 73 percent, negative predictive value of 98 percent, and positive predictive value of 21 percent.19
Human neutrophil lipocalin (HNL), originating from the neutrophils, has been shown to be useful in sepsis, but, so far, the number of relevant studies is limited. HNL is the same as neutrophil gelatinase-associated lipocalin (NGAL), which is a potential biomarker for predicting acute kidney injury (AKI).20 However, NGAL used for AKI originates from the kidneys. The way to distinguish the origin of NGAL is to look at the form of the molecule. If there is a predominant amount of monomeric and/or heterodimeric NGAL as compared with homodimeric NGAL, that indicates NGAL originating from the kidney; an equal or predominant amount of homodimeric NGAL, as compared with monomeric or heterodimeric NGAL, indicates NGAL originating in the neutrophils. (The heteromeric form is NGAL linked to matrix metalloproteinase-9.) It is common practice to call the marker HNL when used in sepsis and NGAL when used in AKI.
Another approach involves the combination of biomarkers. The diagnostic role of PCT and MR-proADM, both in sepsis and in localized infections, together with their contribution to effective antibiotic therapy, has been evaluated. The combined use of PCT and MR-proADM increased the post-test probability of the diagnosis of bacterial infections compared to PCT alone.21 In addition, several studies have investigated the prognostic value of soluble‐triggering receptor expressed on myeloid cells-1 (sTREM-1) in patients with infection. Elevated sTREM-1 concentrations had a moderate prognostic significance in assessing the mortality of infection in adult patients. However, sTREM-1 alone is insufficient to predict mortality as a biomarker.22,23
In summary, the continued efforts both to raise sepsis awareness and to improve upon our current diagnostic approaches are of vital importance. As the Sepsis-3 definition points to life‐threatening organ dysfunction caused by a dysregulated host response to infection, the medical community now, more than ever, needs to be vigilant to those cases that present with early and often difficul-to-navigate constellations of signs and symptoms. There must be a paradigm shift in how the individual sepsis patient is viewed, as there are many subgroups that will remain challenging to distinguish based on clinical observation alone. This calls for refinement of diagnostic tools and biomarkers that can aid clinical decision support as well as better optimize therapeutic intervention for better patient outcomes.
Additionally, making it easier for providers to access the latest in guidelines and research via mobile technologies such as the clinical sepsis guide app will hopefully aid those on the front lines of the battle for better sepsis care and outcomes. If we are to move the needle on sepsis, it will call formultidisciplinary and team-based approaches, working in concert with all stakeholders in the sepsis ecosystem.
REFERENCES
1. CDC Sepsis Data Reports. 2017.
2. Kadri SS, Rhee C, Strich JR, et al. Estimating ten‐year trends in septic shock incidence and mortality in United States Academic Medical Centers Using Clinical Data. Chest. 2017;151(2):278‐285.
3. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013. HCUP. 2016.
4. Marik PE, Taeb AM. SIRS, qSOFA and new sepsis definition. J Thorac Dis. 2017;9(4):943‐945.
5. White Blood Cells. 2018.
6. Wiersinga WJ, Leopold SJ, Cranendonk DR, Van der Poll T. Host innate immune responses to sepsis. Virulence. 2014;5(1):36‐44.
7. Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014:5(1):4‐11.
8. Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576-258.2
9. Ward NS, Levy MM, eds. Sepsis: definitions, pathophysiology and the challenge of bedside management. New York: Humana Press. 2017. eBook ISBN: 978-3-319-48470-9.
10. Vincent JL. Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323‐2329.
11. Sridharan P, Chamber RS. The efficacy of procalcitonin as a biomarker in the management of sepsis: slaying dragons or tilting at windmills? Surg Infect. 2013;14(6):489‐511.
12. Ku Y-H, Chuang Y-C, Yu W-L. Post‐viral sepsis: Clinical experience in post‐viral influenza co‐infections. SMGroup. 2016.
13. Lee SM, An WS. New clinical criteria for septic shock: serum lactate level as new emerging vital sign. J Thorac Dis. 2016;8(7):1388‐1390.
14. Mitchell AB, Tang B, Shojaei M, et al. A novel sampling method to detect airborne influenza and other respiratory viruses in mechanically ventilated patients: a feasibility study. Ann Intensive Care. 2018;8(1):45.
15. Engelmann I, Dubos F, Lobert PE, et al. Diagnosis of viral infections using myxovirus resistance protein A (MxA). Pediatrics. 2015;135(4):e985-93.
16. Lease ED, Alexander DB. Fungal diagnostics in pneumonia. Semin Respir Crit Care Med. 2011;32(6):663–672.
17. Theel ES, Doem CD. β-d-glucan testing is important for diagnosis of invasive fungal infections. J Clin Microbiol. 2013;51(11):3478‐3483.
18. Srisanthong P, Wongsa A, Kittworawitkul P, Wattanathum A. Early IL‐6 response in sepsis is correlated with mortality and severity score. Crit Care. 2013;17(Suppl 2):P34.
19. Morsy AA, Elshall LY, Zaher MM, Abd Elsalam M, Nassr AE. CD64 cell surface expression on neutrophils for diagnosis of neonatal sepsis. Egypt J Immunol. 2008;15(2):53‐61.
20. Adib-Conquy M, Cavaillon JM. Compensatory anti‐inflammatory response syndrome. Thromb Haemost. 2009;101(1):36‐47.
21. Zhang A, Cai Y, Peng-Fei W, et al. Diagnosis and prognosis of neutrophil gelatinase‐associated lipocalin for acute kidney injury with sepsis: a systematic review and meta‐analysis. Crit Care. 2016;20:41.
22. Angeletti S, Spoto S, Fogolari M, et al. Diagnostic and prognostic role of procalcitonin (PCT) and MR‐pro‐Adrenomedullin (MR‐proADM) in bacterial infections. APMIS. 2015; 123(9):740‐817.
23. Su L, Liu D, Chai W, Liu D, Long Y. Role of sTREM‐1 in predicting mortality of infection: a systematic review and meta‐analysis. BMJ Open. 2016;6(5):e010314.
About the Author

Joseph Chiweshe, MD, MPH
is Senior Manager; IDN & Evidence Generation for Beckman Coulter’s North America Commercial Operations (NACO) Strategy & Partnership marketing team. Dr. Chiweshe was formerly a Global Medical Innovations Manager for Beckman Coulter’s Strategy and Innovation team where he was responsible for leading long-term transformational opportunities.