Clinical, operational, and financial perspectives of POC diabetes testing
Earning CEUs:
Diabetes is a complex, chronic illness that requires continuous medical care beyond glycemic control. Ongoing patient education and support are critical to preventing costly acute complications and reducing the risk of long-term complications that can significantly decrease quality of life.
Point-of-care testing (POCT) is one tool that can improve opportunities to educate patients and positively impact outcomes. This article examines advances in POC HbA1c standardization, and key performance indicators, including practice efficiency, outcomes, and patient satisfaction. Finally, perceived barriers to implementing a POCT program are discussed, and in that context quality control, operational, and financial concerns are addressed.
The 2018 American Diabetes Association (ADA) Standards of Medical Care in Diabetes state that the use of point-of-care HbA1c testing may provide an opportunity for more timely treatment changes during encounters between patients and providers.1 To appreciate the clinical value of diabetic testing at the point-of-care, it is critical to understand the increasing burden diabetes is placing on patients and healthcare costs, as well as the positive impact of maintaining glycemic control.
The high cost of diabetes
Diabetes is a treatable disease and yet is among the major causes of death in most developed countries. It is one of the largest health emergencies of the 21st century, and one of four priority non-communicable diseases targeted for action by world leaders.2
The global prevalence of diabetes has nearly doubled since 1980, rising from 4.7 percent to 8.5 percent, and it is steadily increasing.2 The 2017 National Diabetes Statistics Report estimated that in the United States 30.3 million people, or 9.4 percent of the U.S. population, have diabetes. This number includes the estimated 7.2 million people who are undiagnosed. The percentage of adults with diabetes increases with age, reaching a high of 25.2 percent among those aged 65 years or older.3
Risk factors for complications of diagnosed diabetes include smoking, obesity, physical inactivity, high blood pressure, high cholesterol, and high blood glucose.4 With clear links to multiple comorbidities such as cardiovascular disease, kidney disease, infections, malignancy, and functional impairment, diabetes places a tremendous financial burden on both patients and healthcare systems.
Total medical costs and lost work and wages for people with diagnosed diabetes in the U.S. is $245 billion. Average medical expenditures among diagnosed diabetics were about 2.3 times higher than expenditures for people without diabetes.3
Self-monitored blood glucose testing
Integrating self-monitored blood glucose (SMBG) results into diabetes management can be a useful tool for guiding medical nutrition therapy and physical activity. Indeed, major clinical trials of insulin-treated patients have included SMBG as part of multifactorial interventions and demonstrate the benefit of intensive glycemic control on diabetes complications.1 Thus, SMBG remains an integral component of effective therapy for type 1 and some type 2 patients. However, the evidence is insufficient regarding when to prescribe SMBG and how often testing is needed for patients with type 2 diabetes using oral agents.1
Of concern is that many patients who check their blood glucose at least once daily report taking no action when results are high or low. Patients should be taught how to use SMBG data to adjust food intake, exercise, or pharmacologic therapy within prescribed limits to achieve specific glycemic goals.
Glucose testing vs. HbA1c
In the laboratory, measurement of glucose in the plasma of fasting subjects is widely accepted. There are significant advantages to glucose testing, including the existence of inexpensive assays on automated instruments that are available in most laboratories worldwide. However, measurement of glucose in the blood is subject to several limitations, some of which are not widely appreciated.5
Samples for fasting glucose analysis should be drawn after an overnight fast of at least eight hours with no caloric intake. But glucose measurement can be affected by several preanalytical variations:
- The concentration of glucose in the blood can be altered if the patient has not adhered strictly to the eight-hour fast or has fasted for a more prolonged period, and it also can be affected by exercise. The fasting issue is a considerable practical problem, as patients are usually not fasting when they visit the doctor, and it is often inconvenient to return for phlebotomy.
- Glucose concentrations decrease in the test tube by five percent to seven percent per hour due to glycolysis, the breakdown of glucose by enzymes, which releases energy and pyruvic acid.5
- Glucose concentrations can be altered by acute stress. If a patient has trouble finding parking or is frustrated by a long wait at the medical facility, that could affect the numbers.5
In contrast, fasting is not required for HbA1c testing. HbA1c is formed by the nonenzymatic attachment of glucose to hemoglobin. Since the life span of red blood cells is approximately 120 days, HbA1c represents the average glucose concentration over the preceding eight to 12 weeks.5
Landmark studies such as the Diabetes Control and Complications Trial (DCCT) (type 1 diabetes) and the United Kingdom Prospective Diabetes Study (UKPDS) (type 2 diabetes) emphasize the role for HbA1c in diabetes management.1 A link between HbA1c and diabetic complications was confirmed and the need for adequate glycemic control underscored.1
Furthermore, the Kumamoto Study and the UK Prospective Diabetes Study (UKPDS) confirmed that intensive glycemic control significantly decreased rates of microvascular complications in patients with type 2 diabetes. Long-term follow-up of the UKPDS patients showed enduring effects of early glycemic control on most microvascular complications. After 10 years of observational follow-up, patients originally randomized to intensive glycemic control had significant long-term reductions of 13 percent in myocardial infarction and 27 percent reduction in all-cause mortality. Achieving HbA1c targets of < seven percent or 53 mmol/mol has been shown to reduce microvascular complications of diabetes.1
The ADA Standards address frequency of HbA1c testing, noting that it should be performed routinely in all patients with diabetes at initial assessment and as part of continuing care.1 Measurement approximately every three months determines whether patients’ glycemic targets have been reached and maintained. Patients with type 2 diabetes with stable glycemia well within target may do well with HbA1c testing only twice per year. Unstable or intensively managed patients, for example pregnant women with type 1 diabetes, may require testing more frequently than every three months.1
ACR and early warning of kidney disease
In addition to regular HbA1c monitoring, yearly urine albumin-to-creatinine ratio (ACR) tests are recommended for patients with diabetes to identify progression of kidney failure.6
Far more convenient to the patient and healthcare provider than a 24-hour urine collection, an ACR measurement is reported in mg albumin/g creatinine and is widely accepted as an indicator of kidney function. Currently, the National Kidney Foundation defines an ACR range from 30 to 300 mg/g as above normal, and multiple tests in this range over a three-month period suggest a problem.7
The impact of chronic kidney disease (CKD) extends beyond just a diminished quality of life for those affected by the disease; it also represents more than $1 trillion in healthcare costs over the next decade.8 But if CKD is detected early and managed appropriately, the deterioration in kidney function can be slowed and the risk of associated cardiovascular complications reduced.9
Laboratory quality at the point of care
The ability of a POC instrument to most closely replicate the actual HbA1c of any given patient is essential. As new HbA1c POC devices have been introduced to the market, standardization has become increasingly important to ensure integrity and equivalence to conventional lab procedures. Devices should meet quality measures set by the National Glycohemoglobin Standardization Program (NGSP) for clinical results to be considered reliable and accurate.10
Since its development in 1996 under the direction of the American Association for Clinical Chemistry, the NGSP has been the authority in establishing guidelines and protocols for standardizing HBA1c testing for both POC and laboratory instruments to Diabetes Control and Complications Trial (DCCT)-equivalent values. This tight criterion provides practitioners confidence in the accuracy and precision of these devices. Based on the stringent criterion set by the NGSP and its alignment with DCCT results, the ADA endorsed the NGSP and specifically recommends that labs use only methods that have passed NGSP certification.10
Currently, a few of the POC devices on the market have met NSGP criteria for analytical performance, making them acceptable options for POC HbA1c testing.10
Studies support POC HbA1c testing
Clinically, a number of studies have found that POCT was associated with a significant reduction in HbA1c over 12 months. An even more sustainable benefit of POC HbA1c results was demonstrated in a large, retrospective, cross-sectional study. Significant HbA1c improvements associated with POC testing were detectable in the short and long term (Figure 1).11
In addition, POCT including HbA1c testing has been shown to significantly improve clinical operations with cost reductions through improved practice efficiency. Following implementation of POCT, one study reported a 21 percent decrease in tests ordered per patient (P < .0001); a decrease in follow-up phone calls and letters by 89 percent and 85 percent, respectively (P < .0001 and P < .0001); and a 61 percent decrease in patient revisits (P = .0002).12 Estimated testing revenues exceeded expenses by $6.62 per patient, and potential cost savings from improved efficiency were $24.64 per patient. The authors noted that the economic benefits of POCT may be realized in both fee-for-service and global payment environments.12
After implementing POCT for HbA1c, lipid panel, and comprehensive metabolic panel in a primary care practice, another study monitored patient satisfaction using an anonymous survey. On a scale of 1 (poor) to 4 (excellent), the score was 3.96; both the patient comments and results strongly indicated a high level of patient satisfaction with onsite POCT.13
Overcoming perceived barriers
Perceived obstacles to implementing a POCT program have included accountability factors such as quality control, adequate staff training, and oversight for accreditation purposes.14
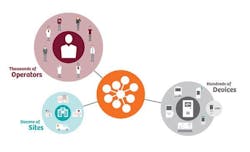
For POCT devices operating under the central laboratory license, the single biggest challenge to the adoption of POCT is maintaining control, regulatory compliance, and training records for thousands of operators performing testing on hundreds of devices in anywhere from 30 to 50 locations within the hospital system.15
In the past, the challenge of maintaining separate POCT data management systems, or middleware, for each manufacturer’s products to interface with the hospital and laboratory information systems (HIS and LIS) has added complexity and increased software licensing costs.16 However, advances include an open-access data management system that can connect more than 160 POC devices, including HbA1c analyzers and glucometers, from all manufacturers (Figure 2).16
A manufacturer-independent solution helps ensure IT investment protection in the event a hospital changes POC equipment vendors at the main hospital or in any spoke hospital, clinic, or physician office across the enterprise.
An open-access data management system can automatically validate and transfer patient results obtained from POCT devices to the electronic medical record to facilitate billing capture and monitor and manage data, POCT devices, and operators. POC coordinators can proactively manage enterprise-wide external quality assessment (EQA) results according to accreditation requirements. Distributions and statistics are easily viewed and filtered with the familiar proactive traffic-light display that flags non-compliances in any connected POCT devices at any site, improving workflow.16
The content of e-learning courses and tests, supplied by each POCT device manufacturer to the open-access data management system, guarantees that only approved content is used for training. When an operator passes the test, indicating successful completion of a course, the results are automatically documented in eLearning, and a message is sent to the data management system, which automatically extends the operator’s certification for another year.16
An open-access data management system is a key enabler for POCT coordinators. It connects devices from any manufacturer and provides operator oversight so testing efficiency is maximized, clinical workflow is improved, compliance is adhered to, and costs are efficiently managed.
POC HbA1c today
Regular HbA1c measurement is recommended by ADA and international guidelines for all patients with diabetes for the assessment of glycemic control by providing information on long-term glycemic status and reliably predicting risk for diabetes-related complications. Accurate, NGSP-standardized POC HbA1c devices are available, and advances in open-access data management can streamline regulatory compliance as well as operator and device management enterprise-wide.
POC HbA1c devices have improved the quality of diabetes management by offering healthcare providers a method for timely assessment of diabetes control, which facilitates informed decision making during consultations. Furthermore, POC HbA1c devices may improve patient adherence and satisfaction by lessening transportation and cost barriers associated with extra office and laboratory visits.
References
- American Diabetes Association. Glycemic targets: standards of medical care in diabetes-2018 Diabetes Care 2018;41(Suppl. 1):S55–S64.
- World Health Organization. Global report on diabetes. April 7, 2016.
- Centers for Disease Control and Prevention. National diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017.
- NIH National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes, heart disease, and stroke.
- Sacks, DB. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518-523.
- The National Kidney Foundation. Diabetes and kidney disease (Stages 1-4).
- The National Kidney Foundation. ACR.
- Scottish Kidney Federation. Chronic kidney disease.
- World Kidney Day: Chronic kidney disease.
- Whitley HP, Yong EV, Rasinen C. Selecting an A1C point-of-care instrument. Diabetes Spectrum. 2015;28(3):201-208.
- Petersen JR, Finley JB, Okorodudu AO, Mohammad AA, Grady JJ, Bajaj M. Effect of point-of-care on the maintenance of glycemic control as measured by A1C. Diabetes Care. 2007;30(3):713-715.
- Crocker JB, Lee-Lewandrowski E, Lewandrowski N, Baron J, Gregory K, Lewandrowski K. Office efficiency: implementation of point-of-care testing in an ambulatory practice of an academic medical center. Am J Clin Pathol. 2014;142:640-646.
- Crocker B, Lee-Lewandrowski E, Lewandrowski N, Gregory K, Lewandrowski K.
Patient satisfaction with point-of-care laboratory testing: report of a quality improvement program in an ambulatory practice of an academic medical center. Clinica
Chimica Acta. 2013;424:8-11. - Shaw JL. Practical challenges related to point of care testing. Practical Laboratory Medicine. 2015;4:22-29.
- Nichols JH. Point of care testing. Clin Lab Med. 2007;27(4):893-908, viii.
- Mardis C, Gundler D. Keeping up with POCT regulatory compliance. MLO. 2017;49(11):24-26.